Announcements #

Today #
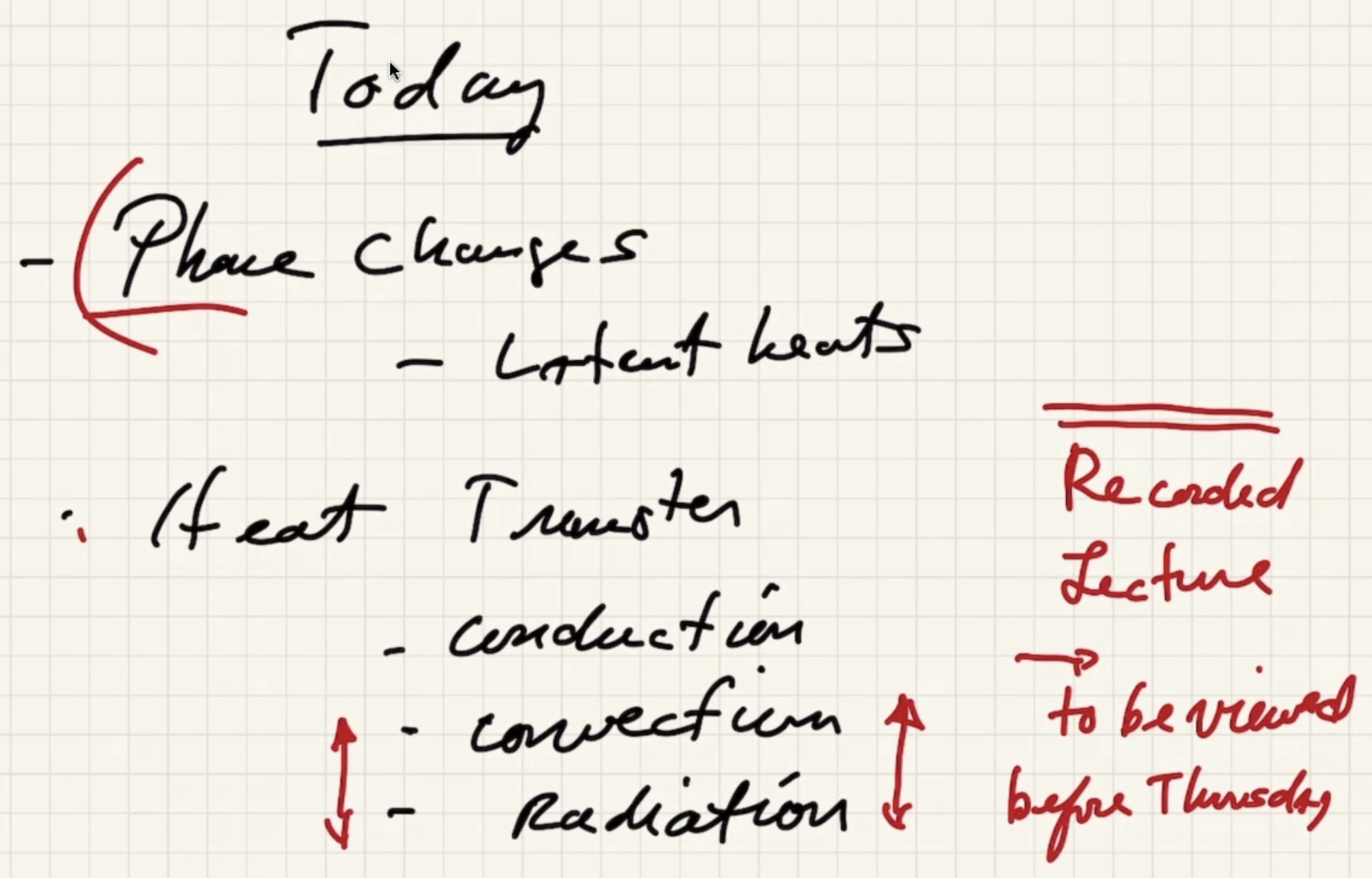
Discussion #
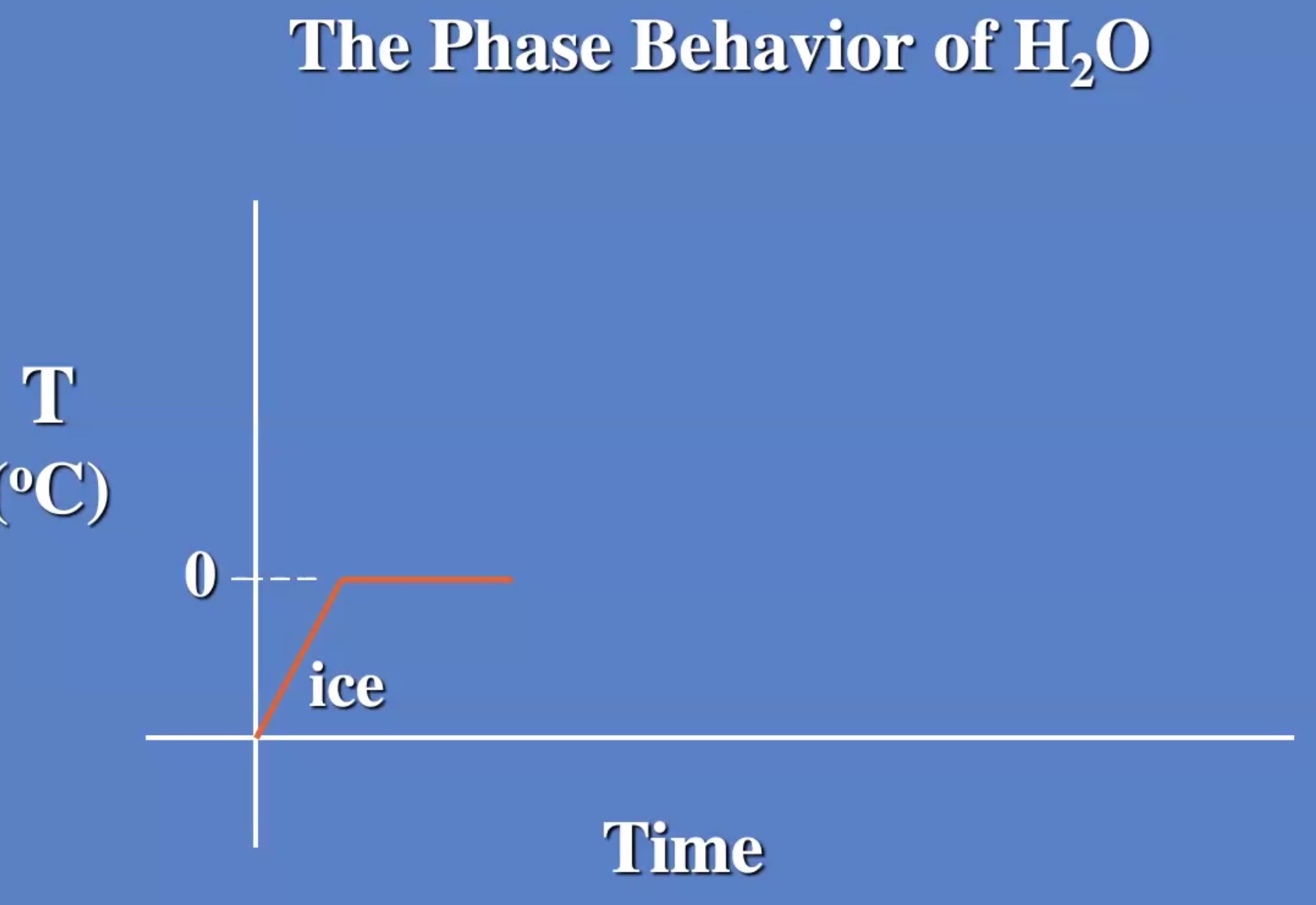
The melting process is breaking the bonds
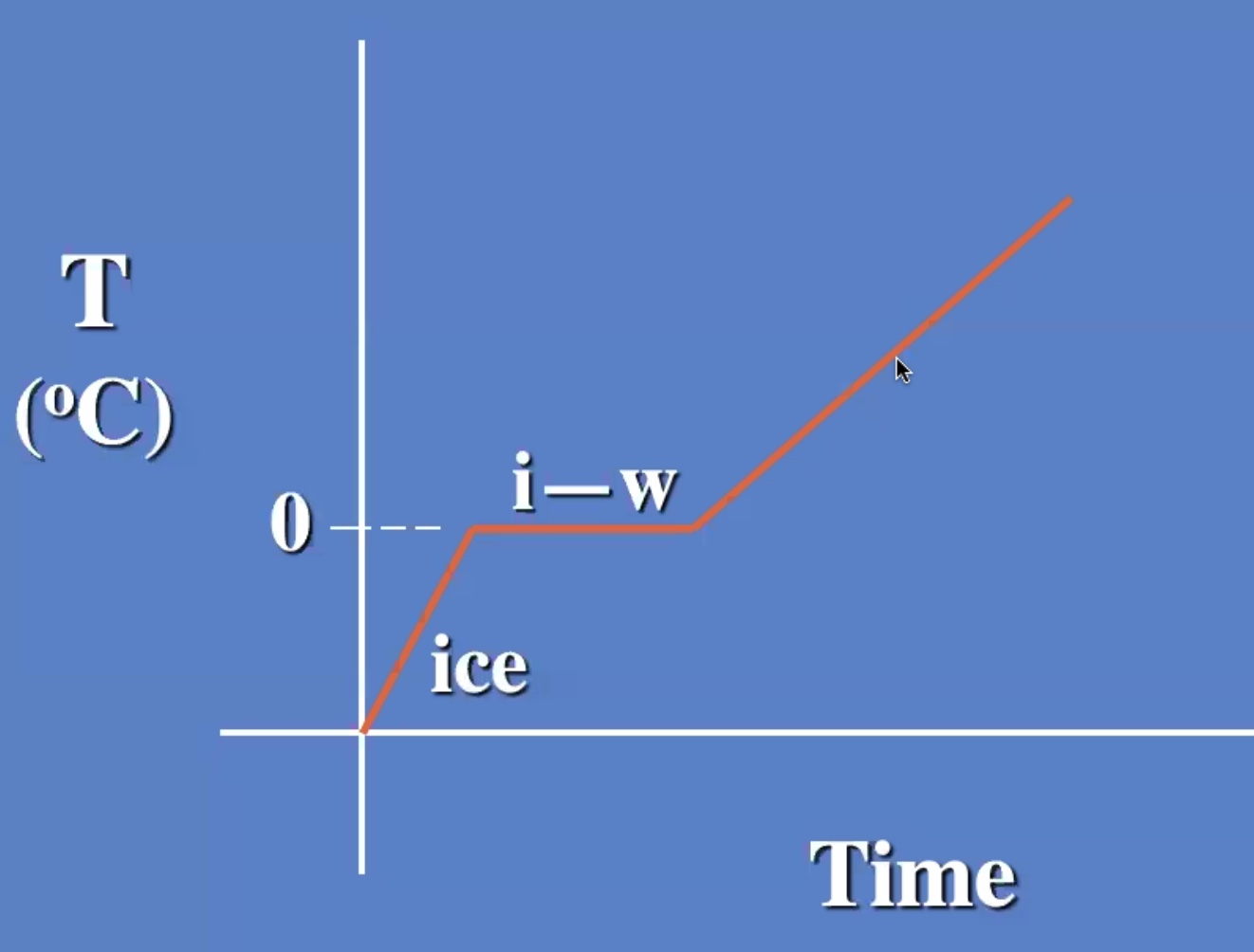
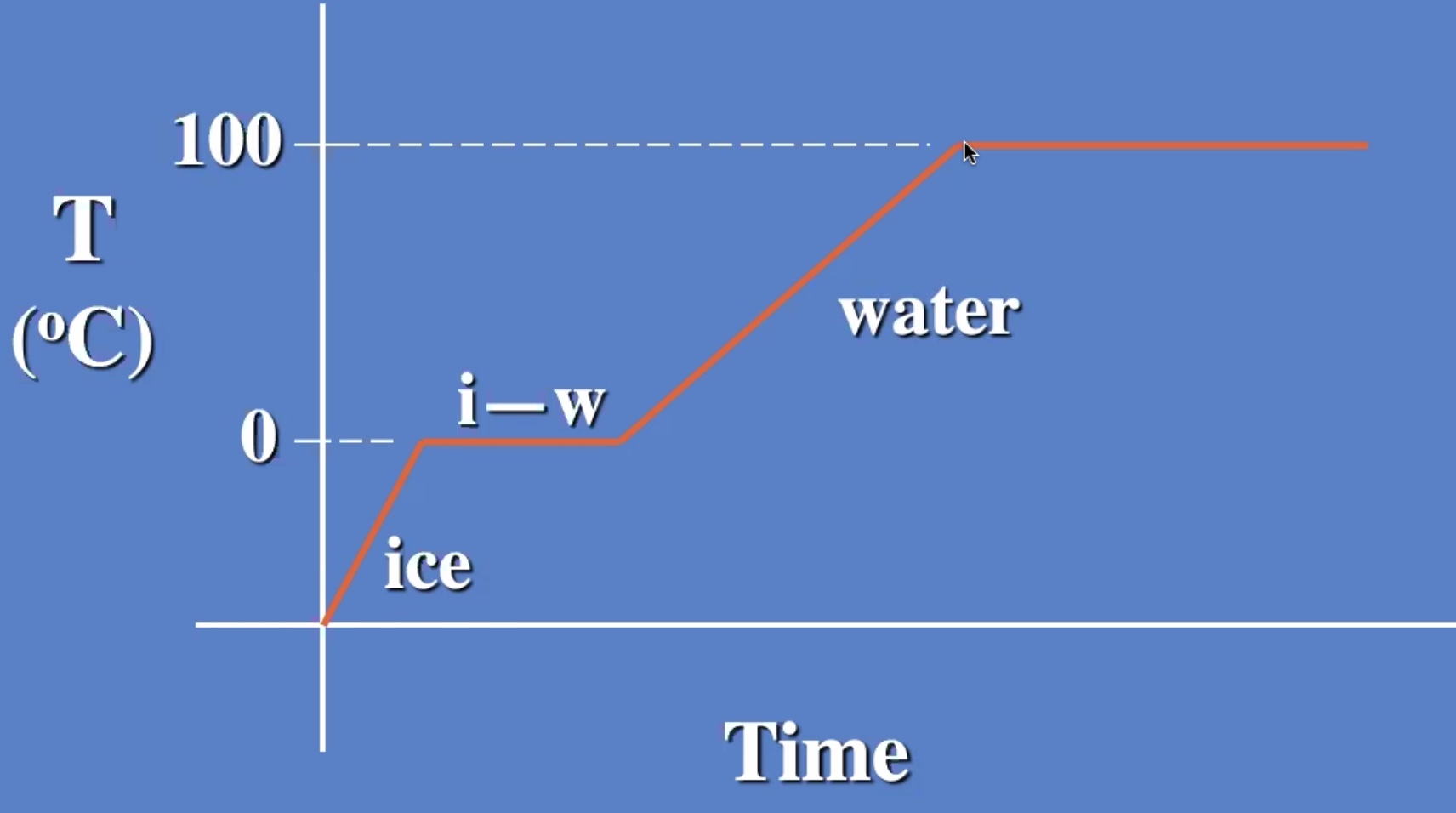
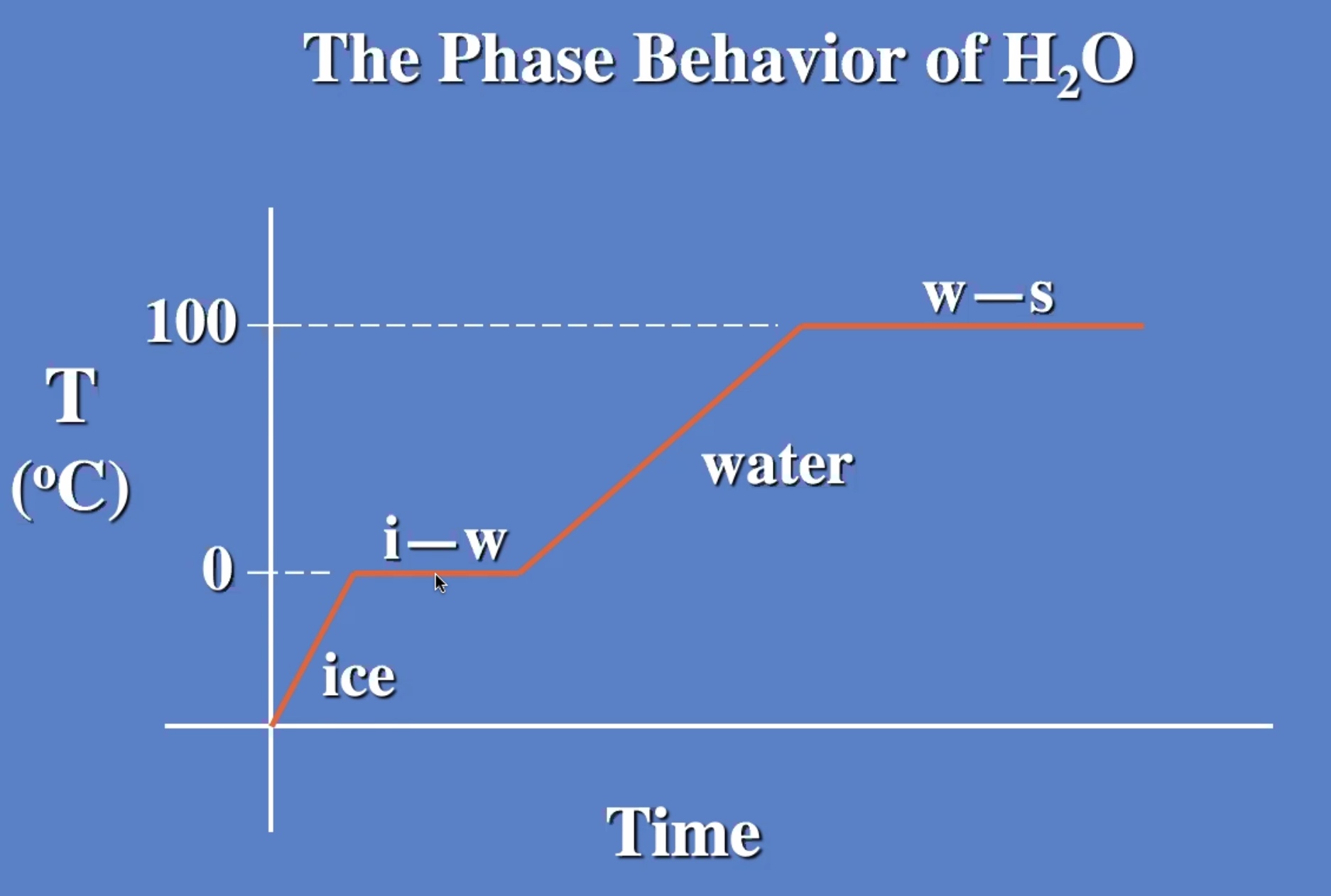
The temp stays the same while changing from liquid to gas also.
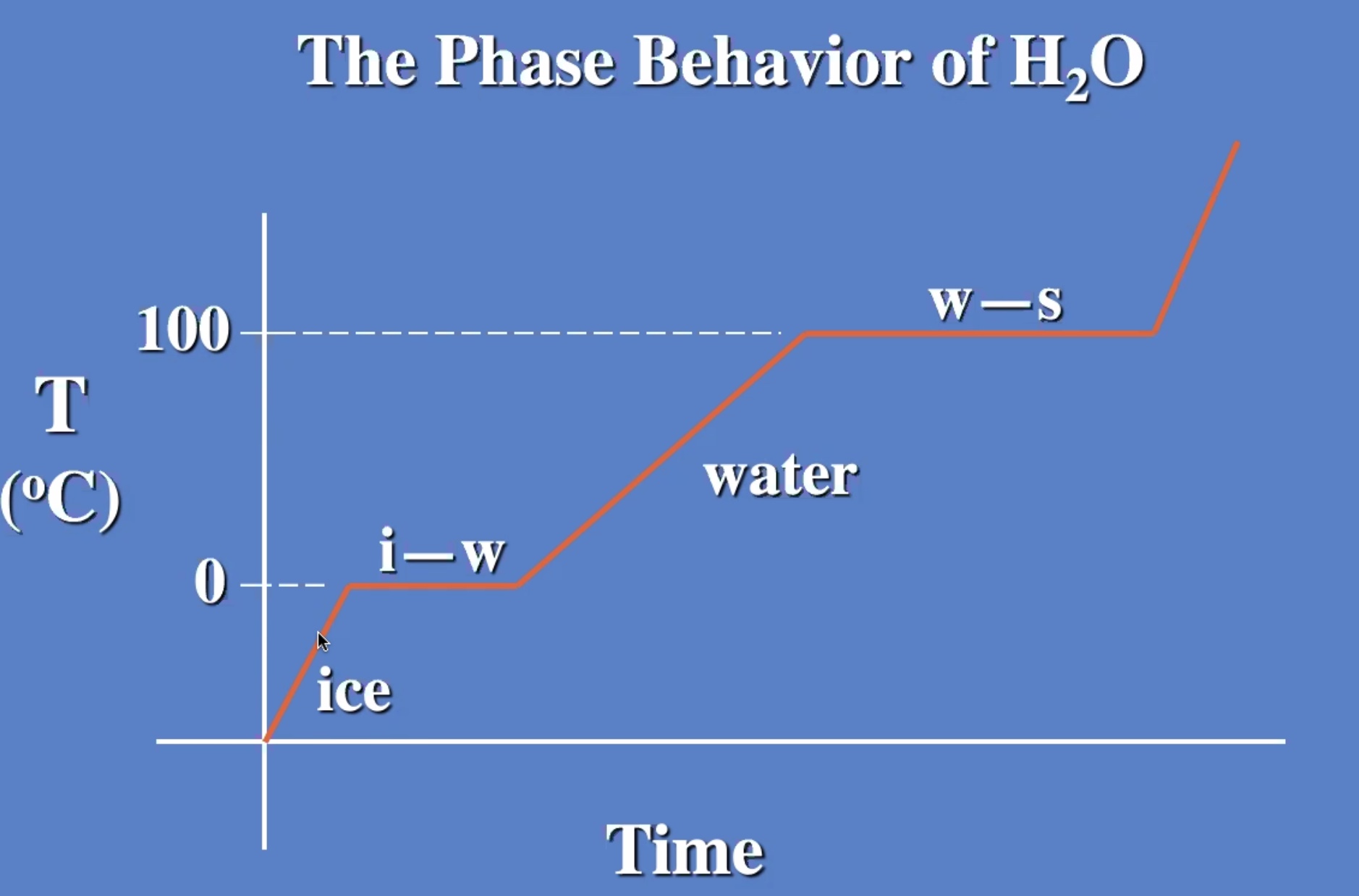
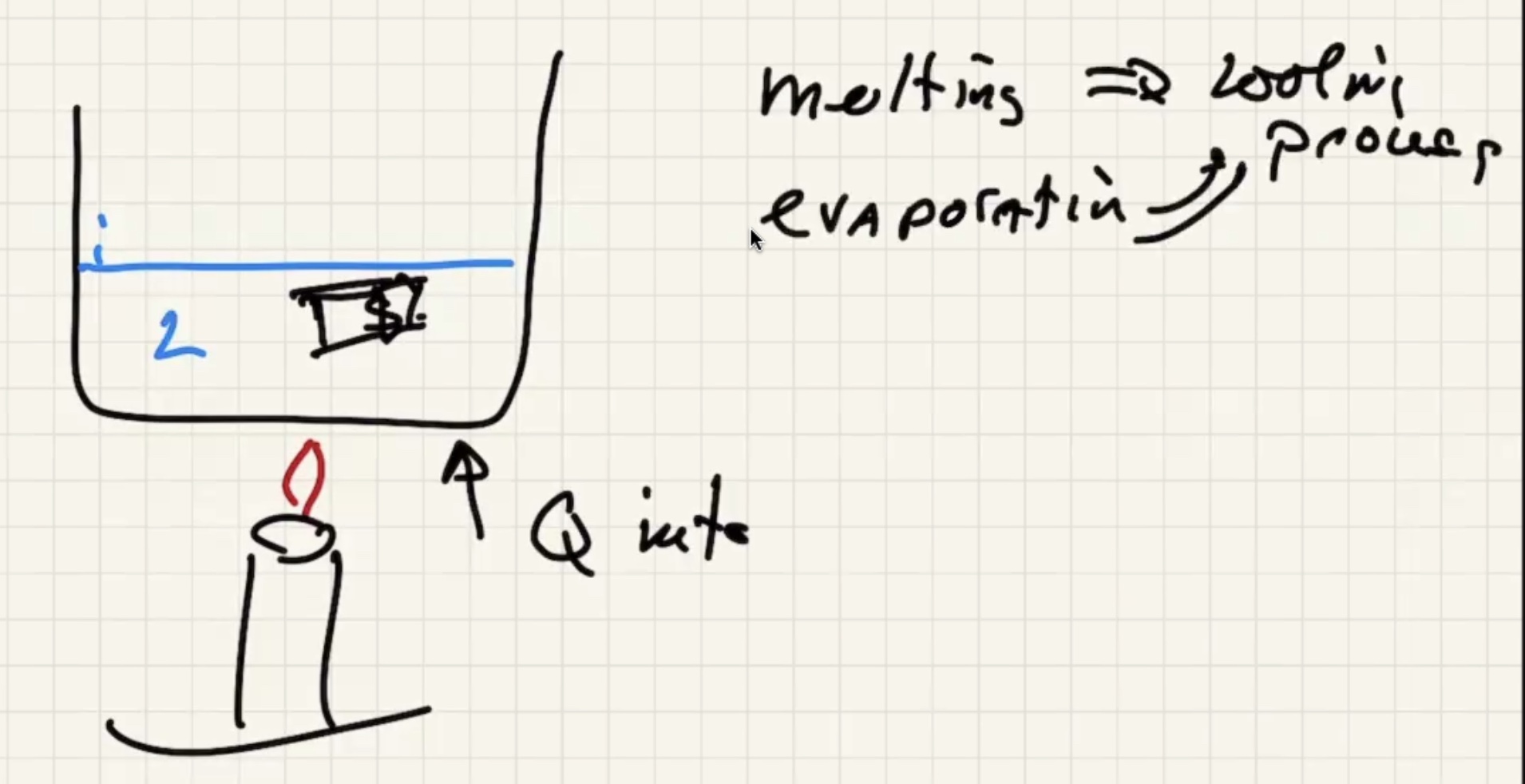
Melting and evaporating are cooling processes.

Freezizng and condensating are warming processes.




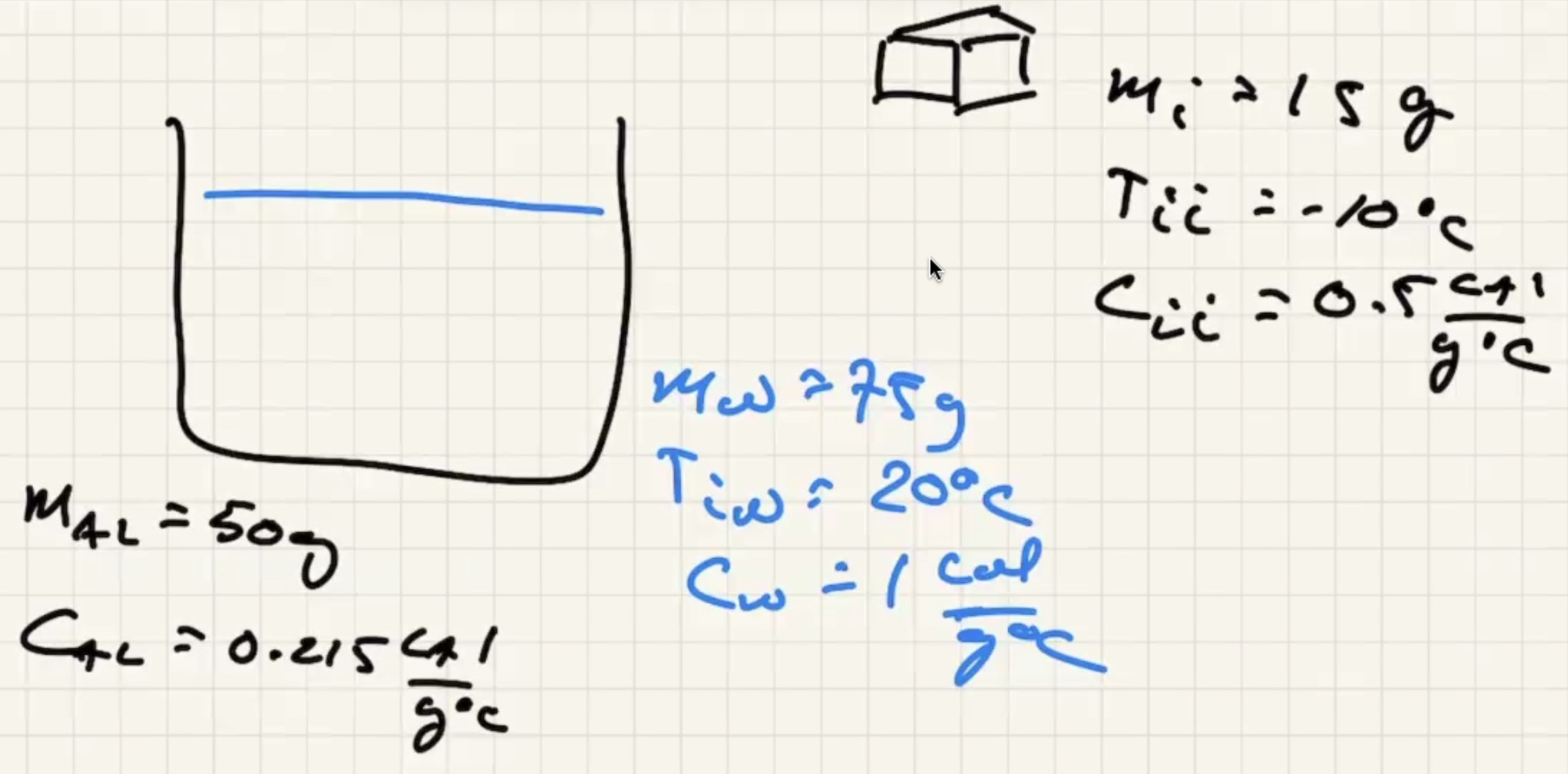
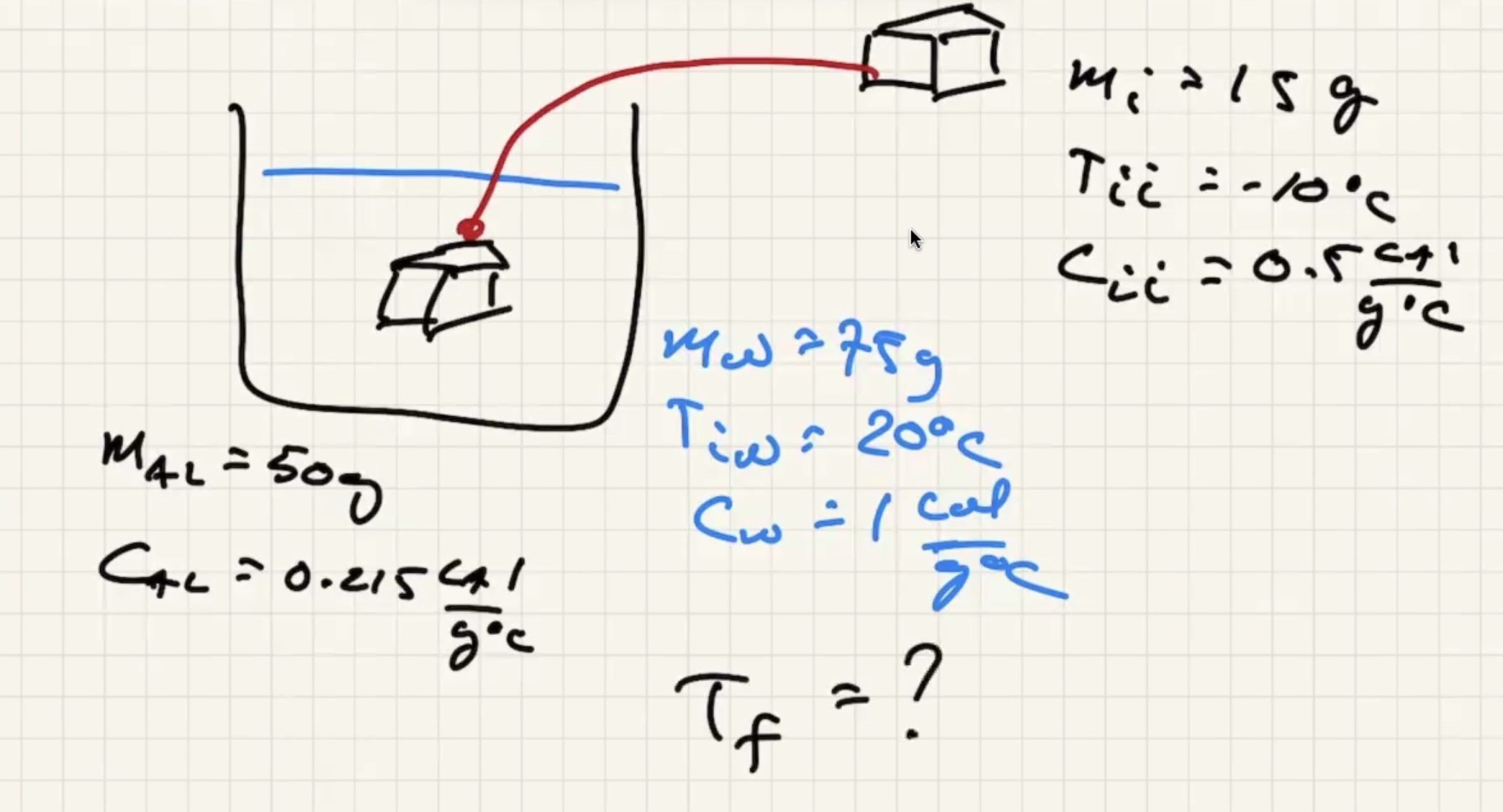
The system is the ice, aluminum, and the water. Isolated from the environment.
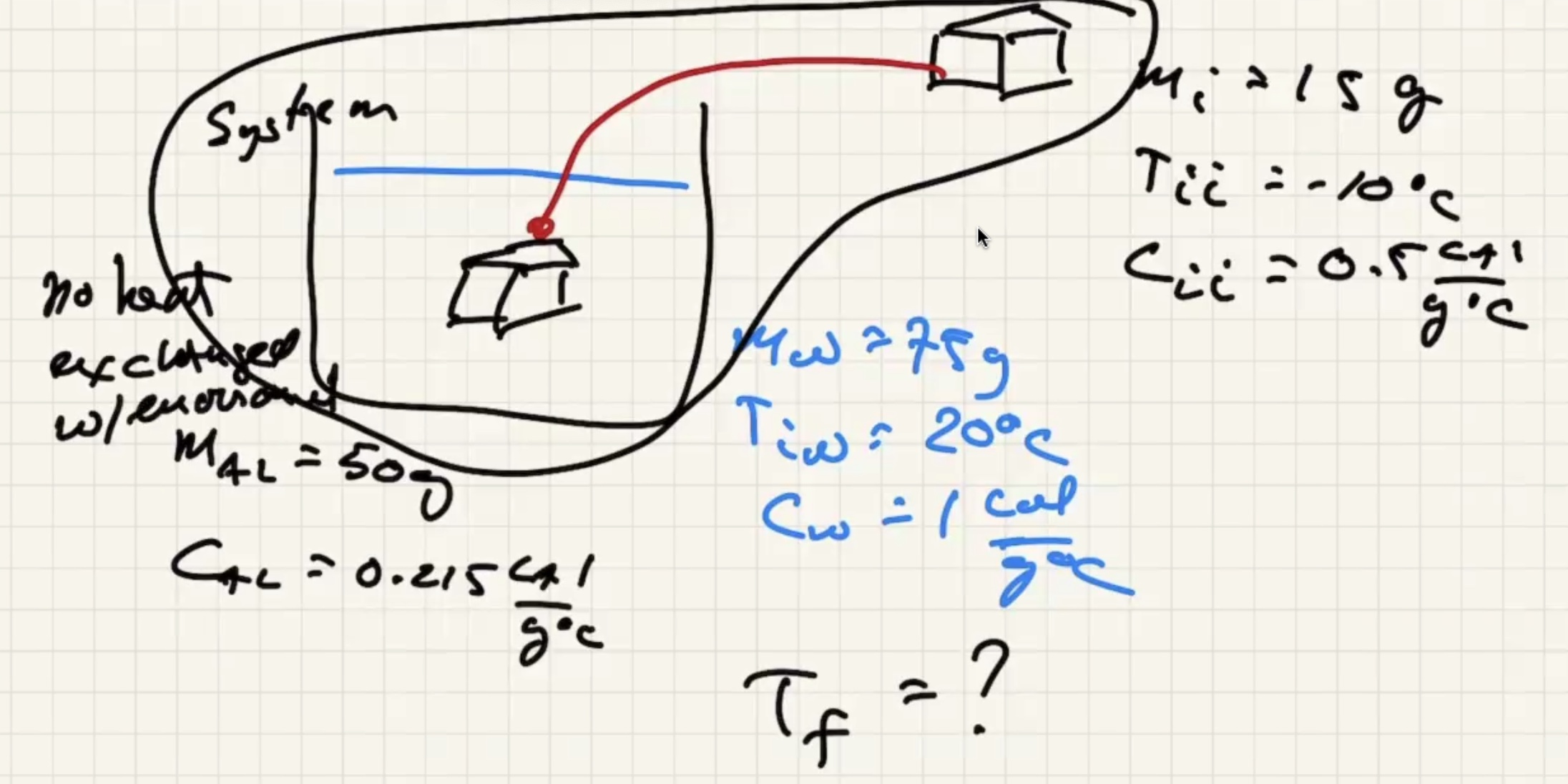
There are three possible cases:

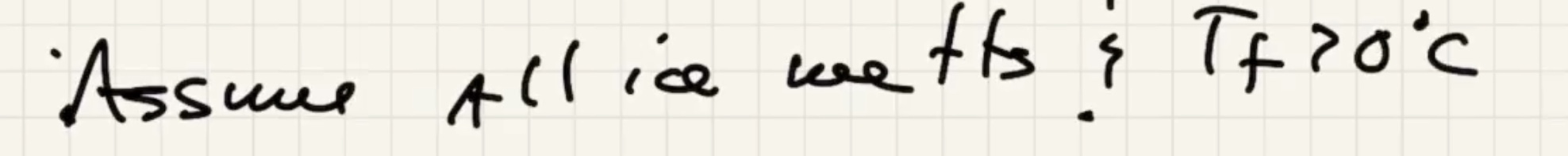
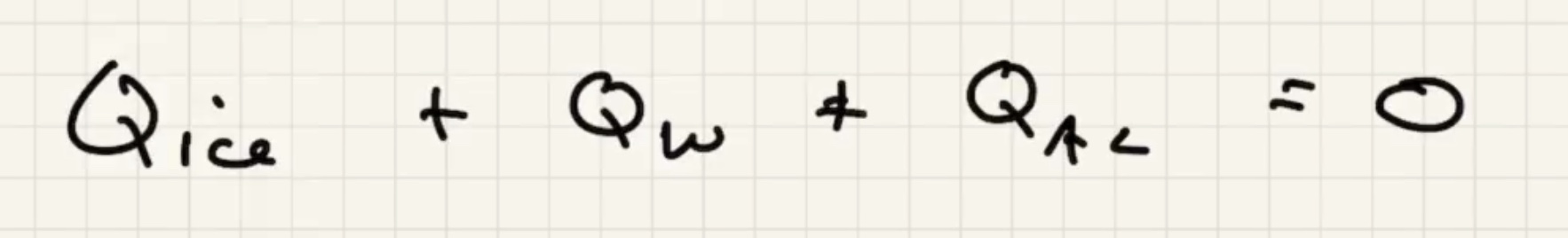
We know the water and liquid will cool, but we’re letting the sign take care of it.
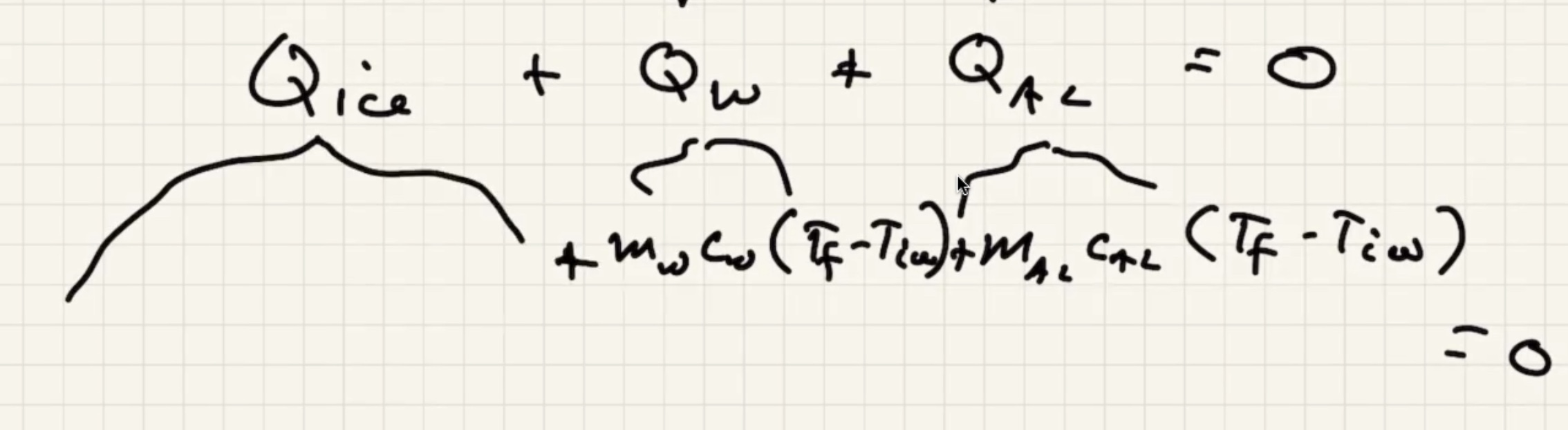
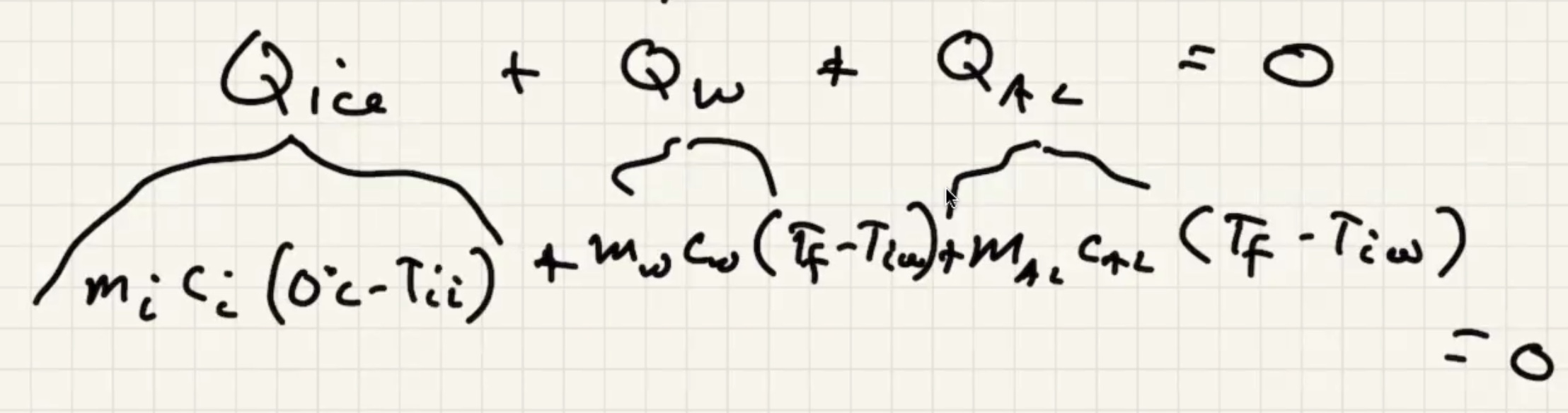
The ice warms to 0 degrees c then starts to melt. And the liquid warms.
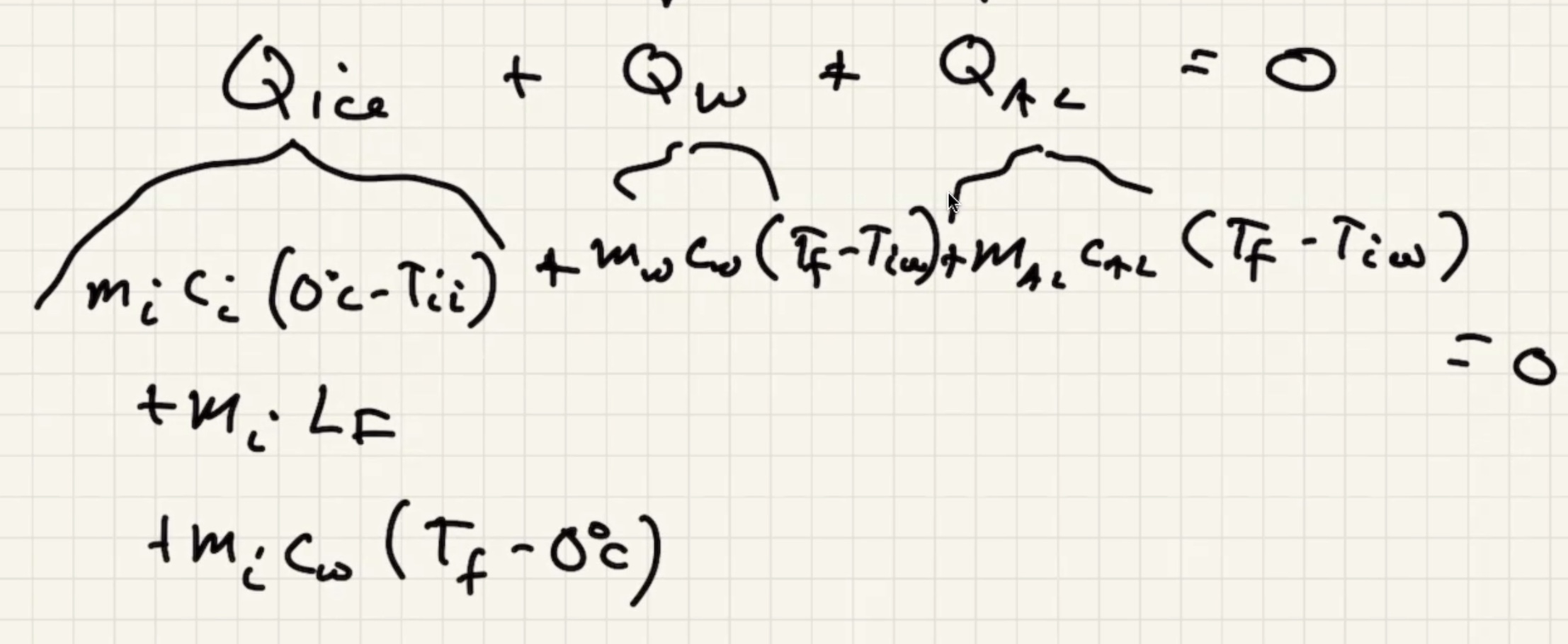
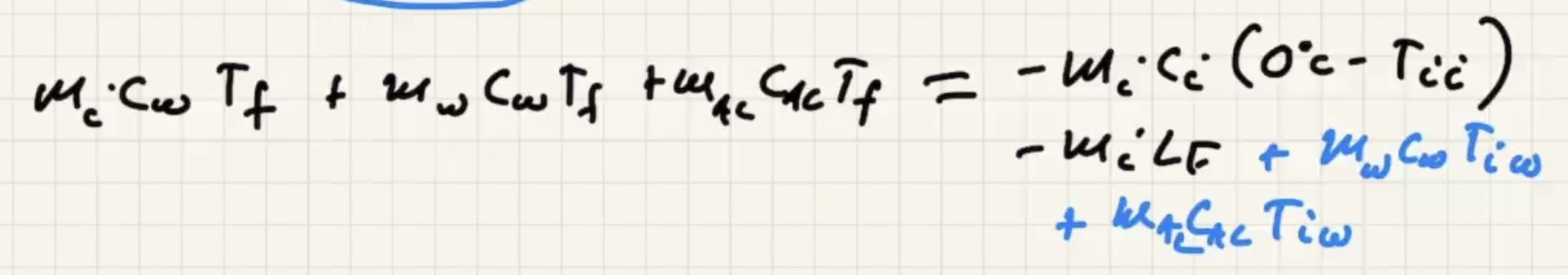
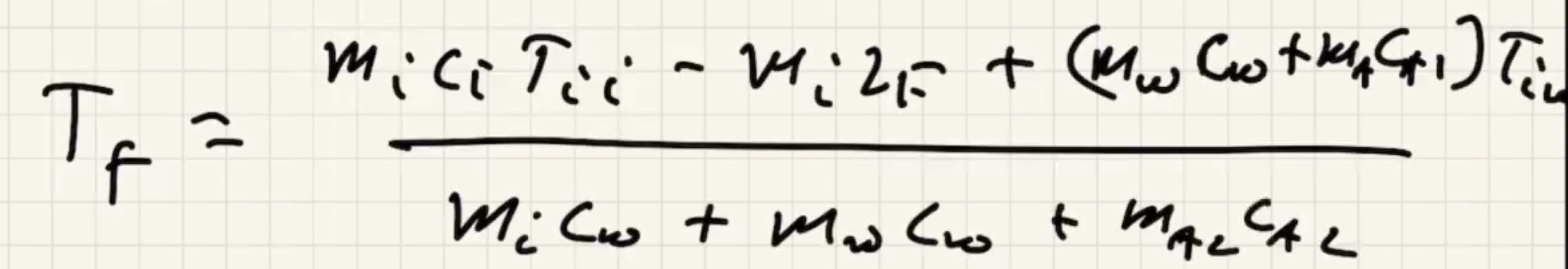
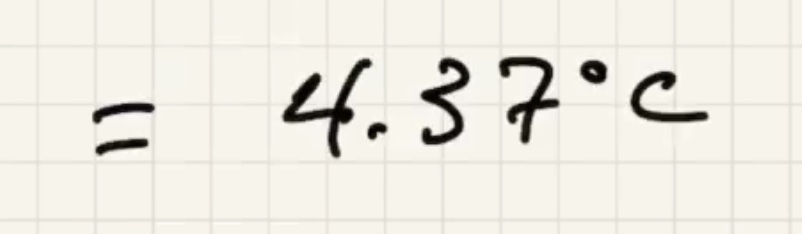
The way that heat flows is the sign of the term, if positive its assuming its flowing in:
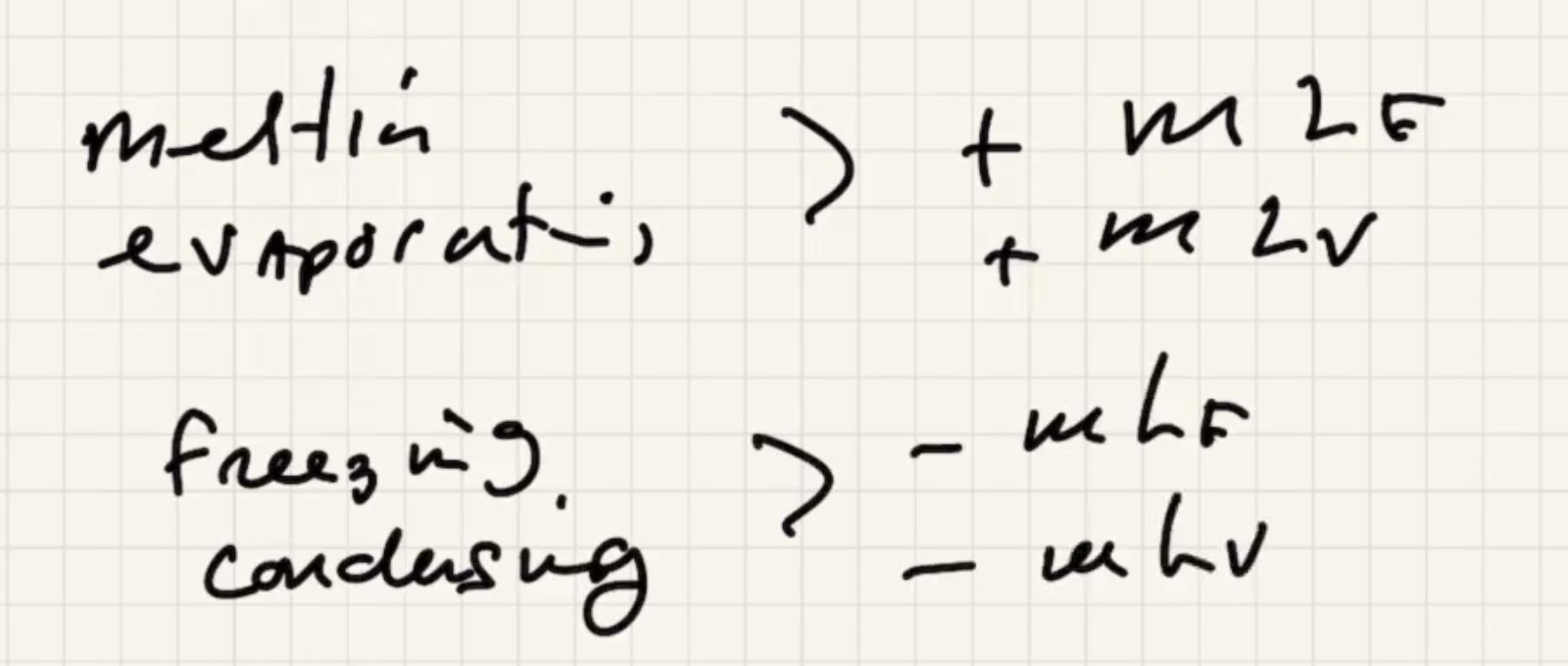




Lets look at conduction in a solid:
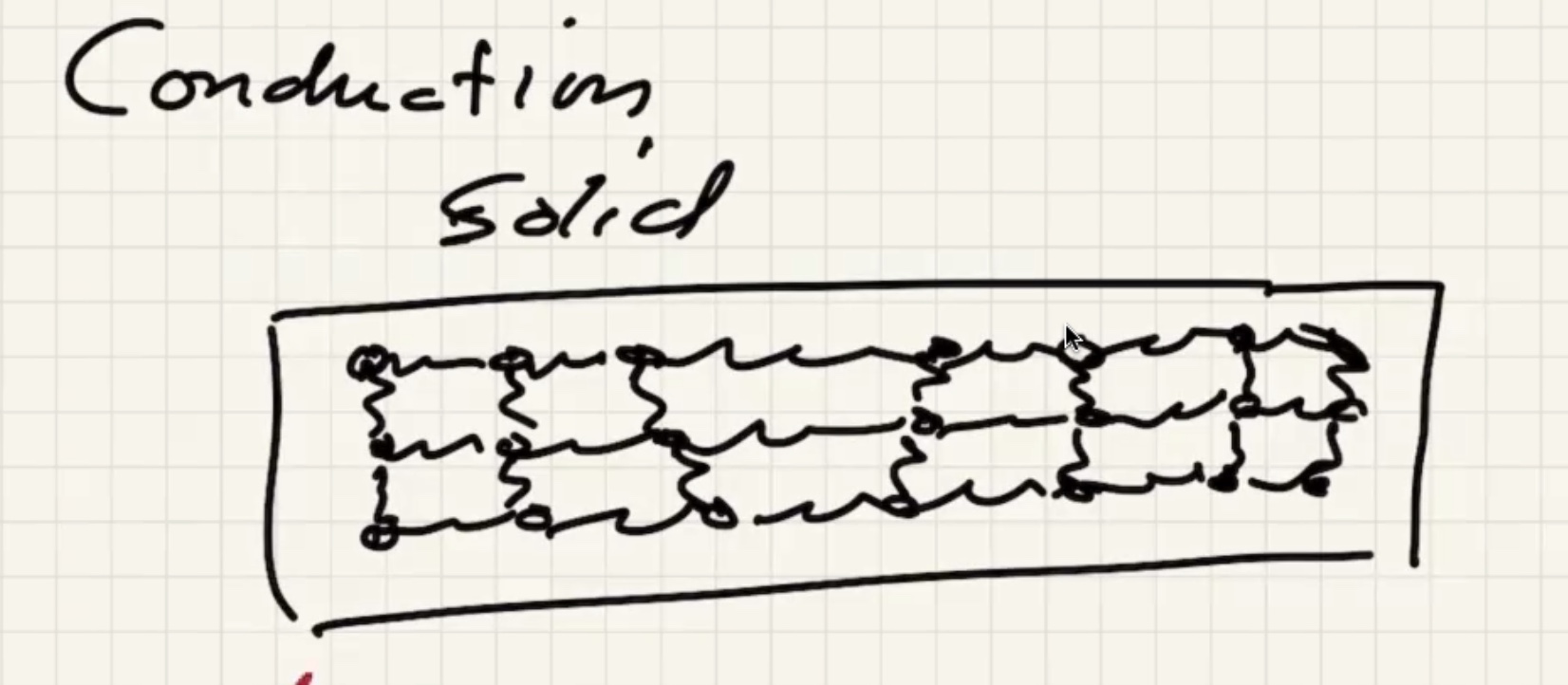
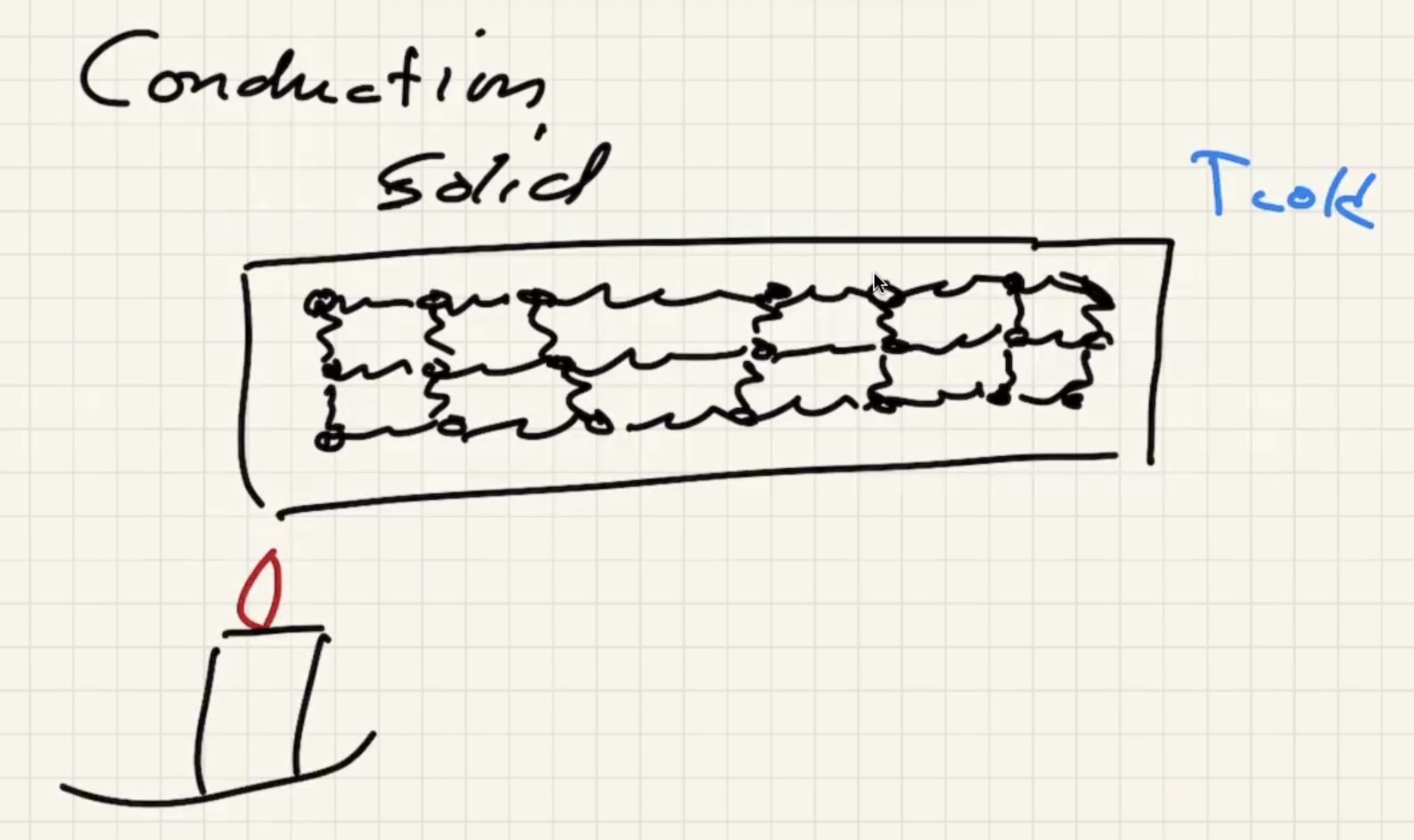
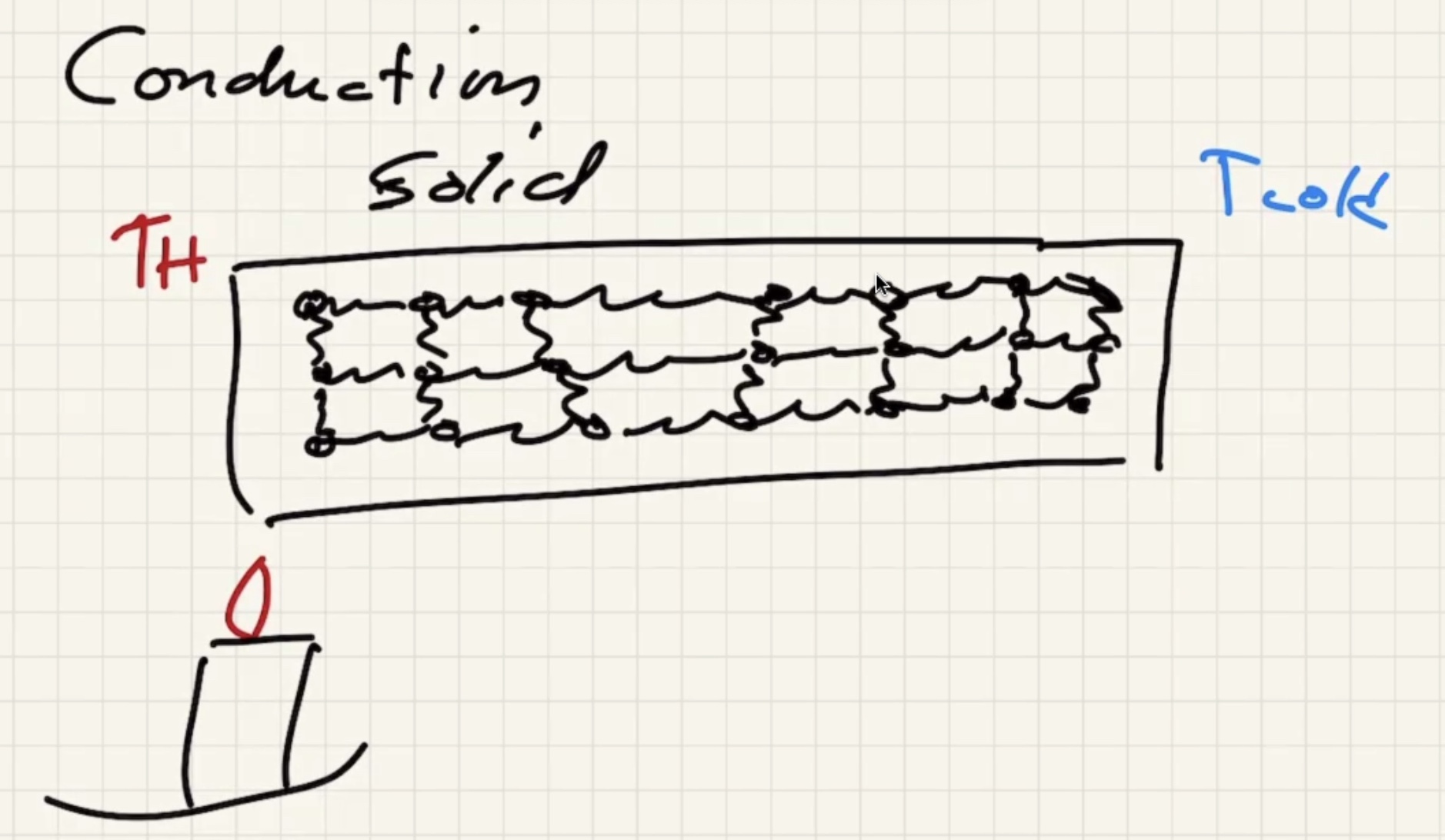
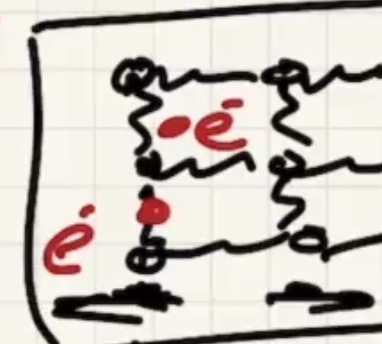
As the solid warms, the molecules start to vibrate.

The energy will start to transfer via lattice vibrations. Solids tend to be better conductors because of the vibrations.

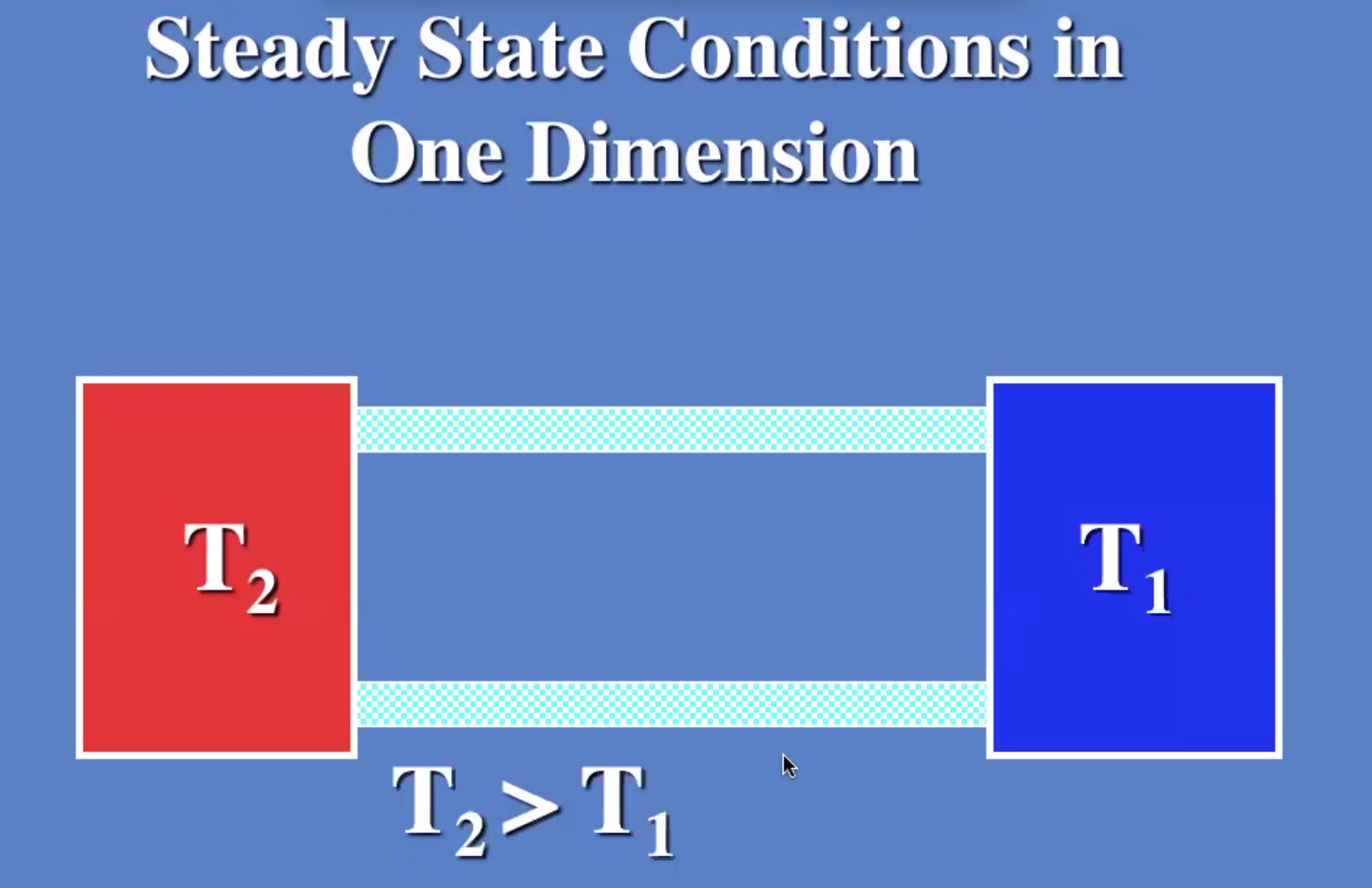
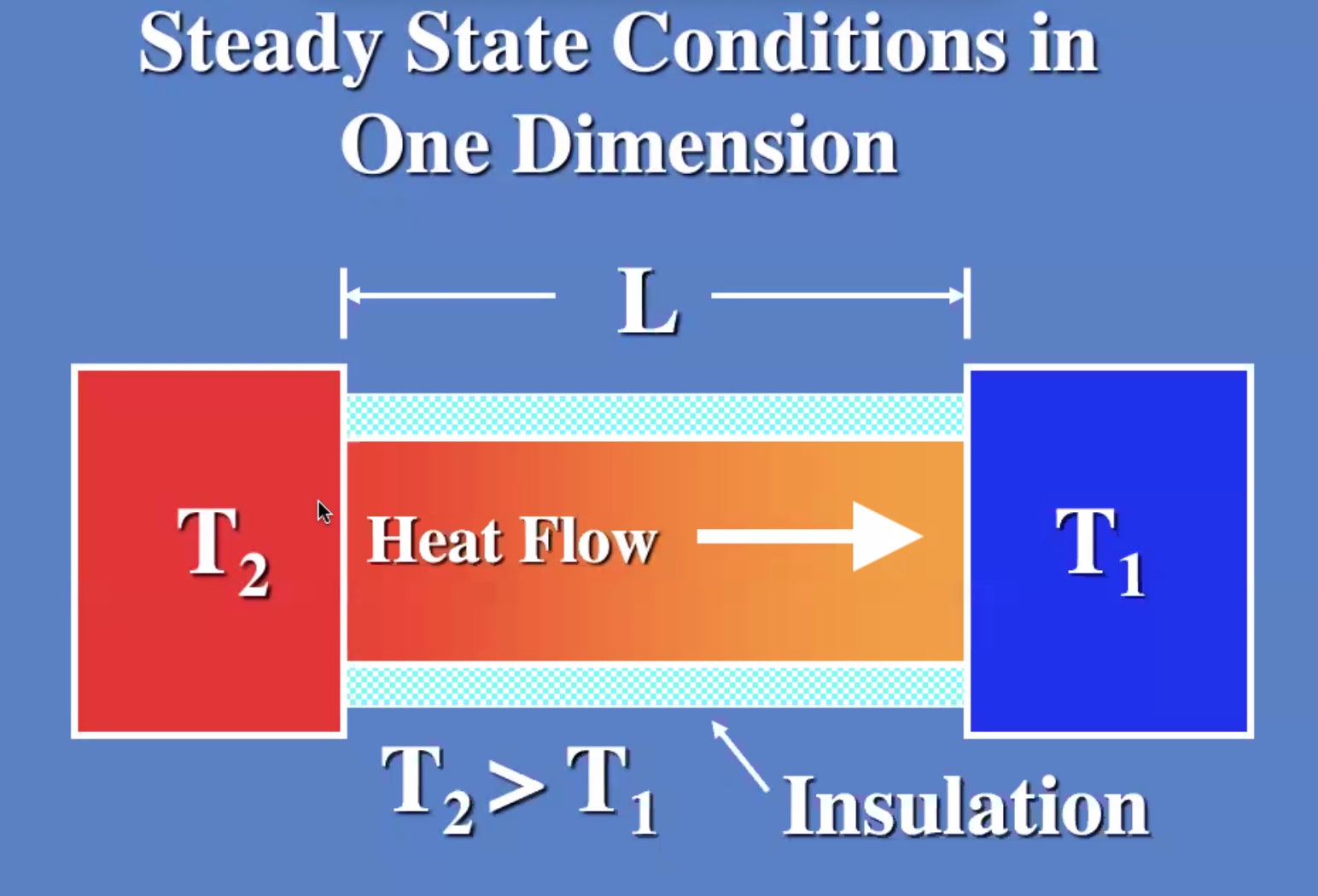



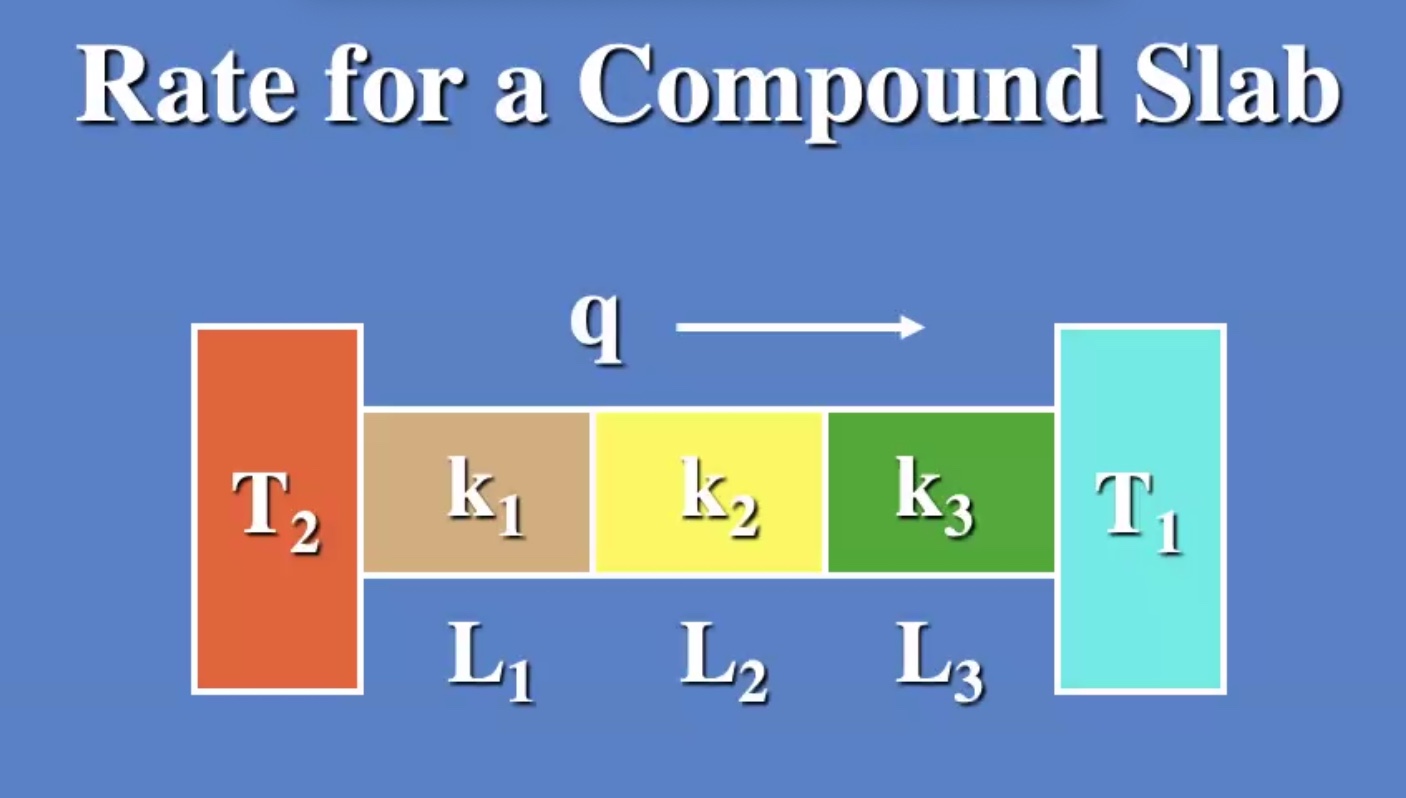
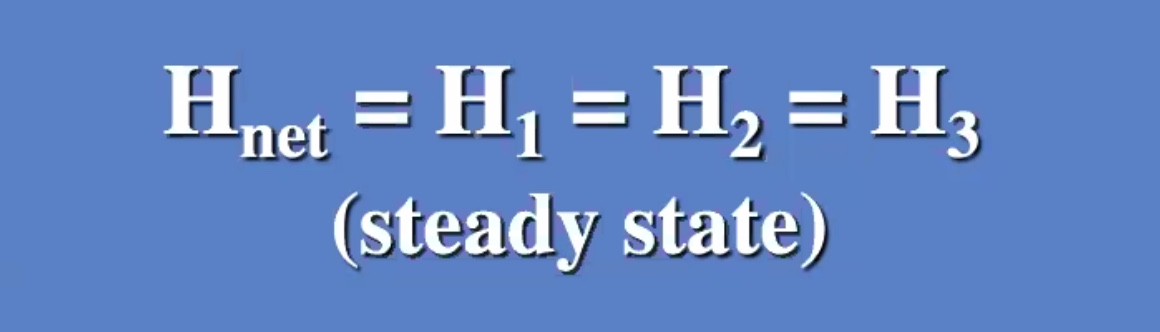
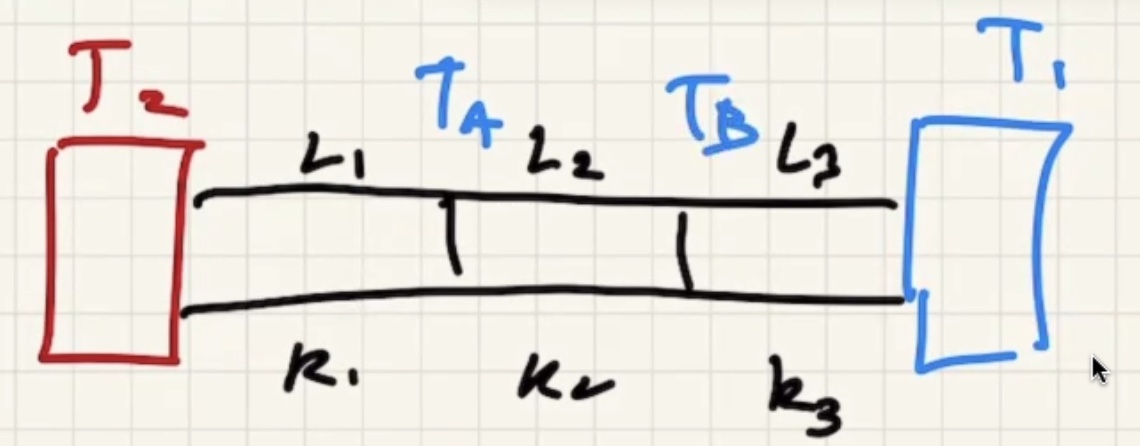
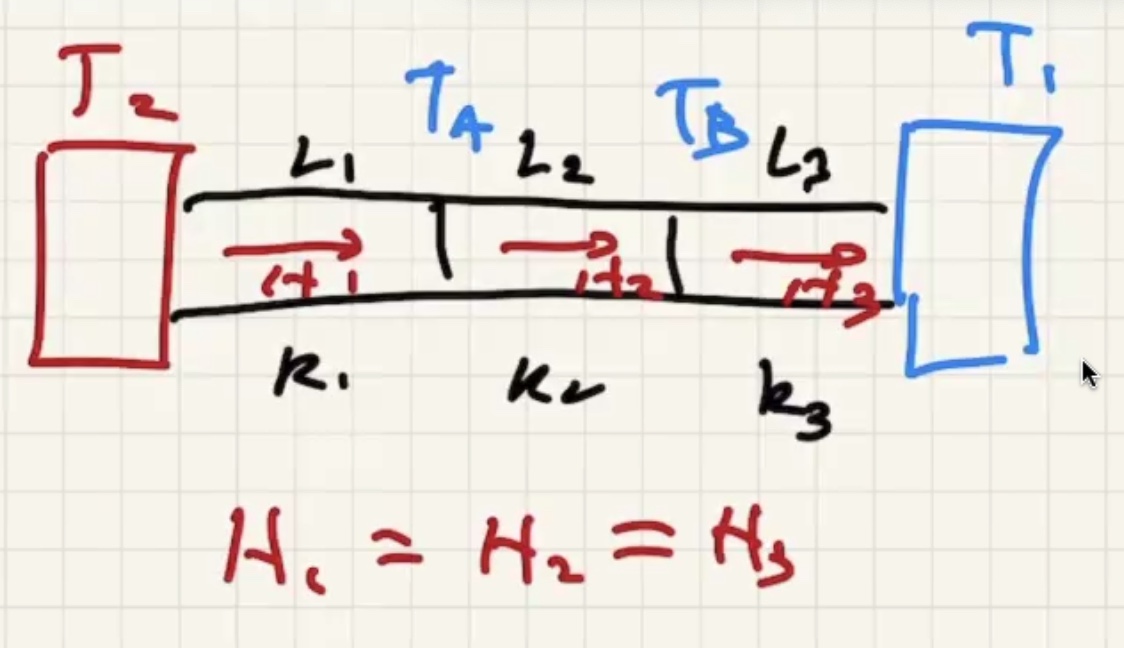
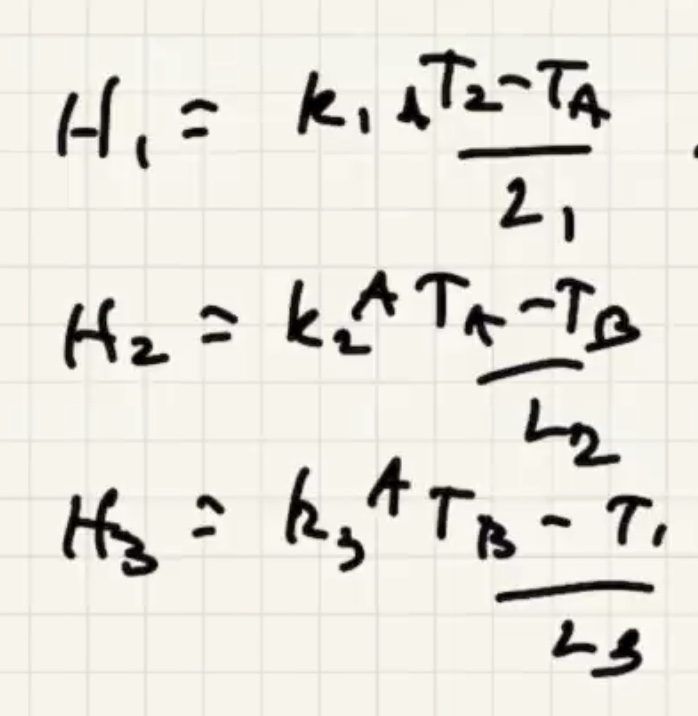
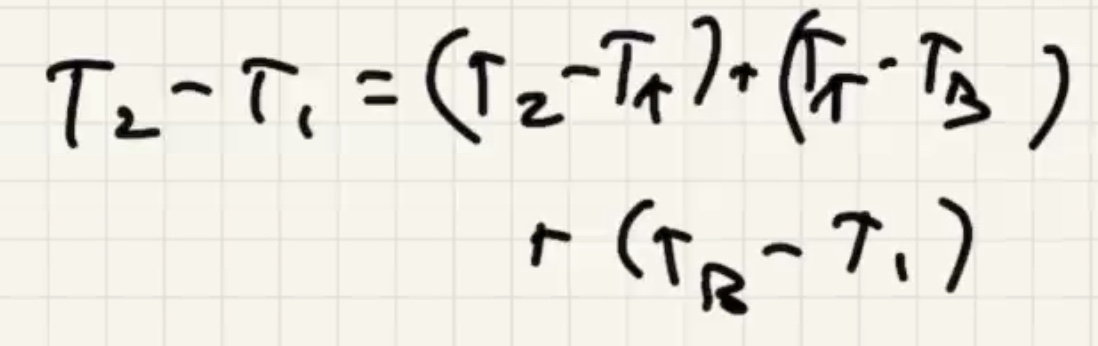
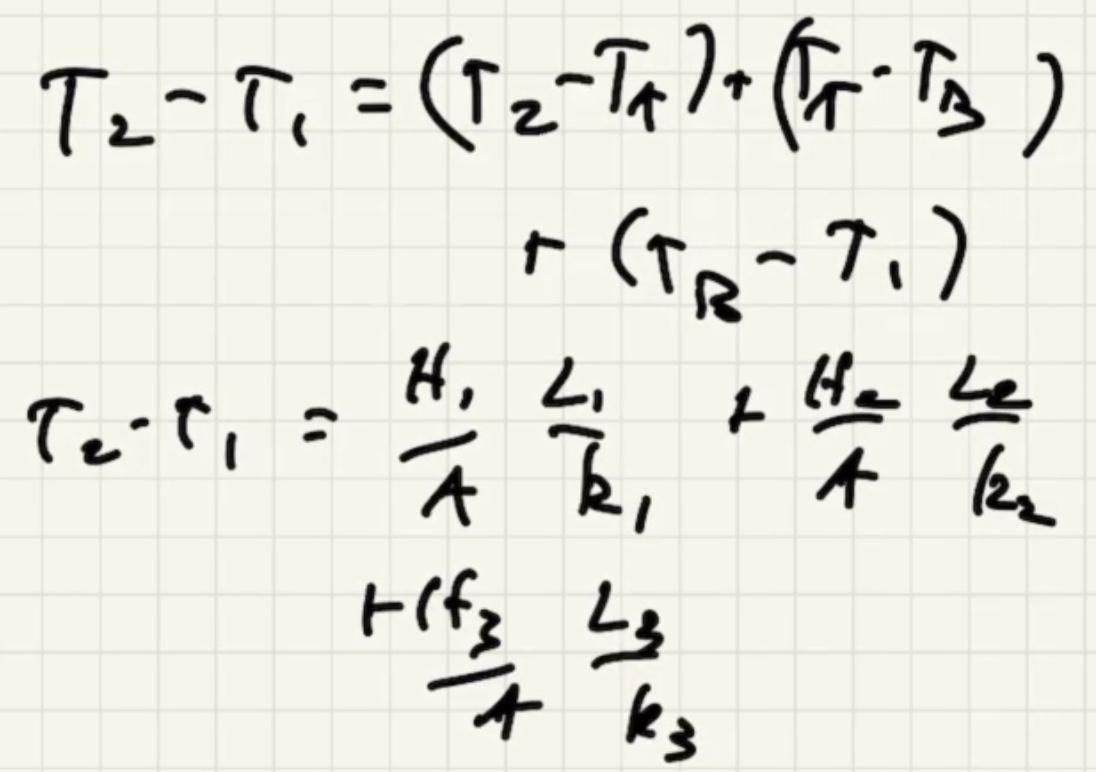
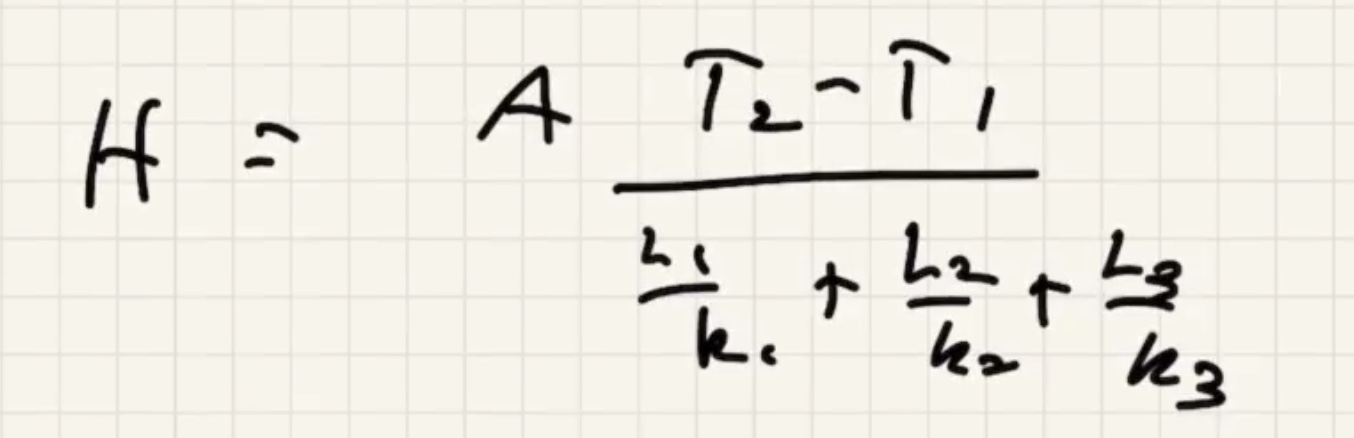
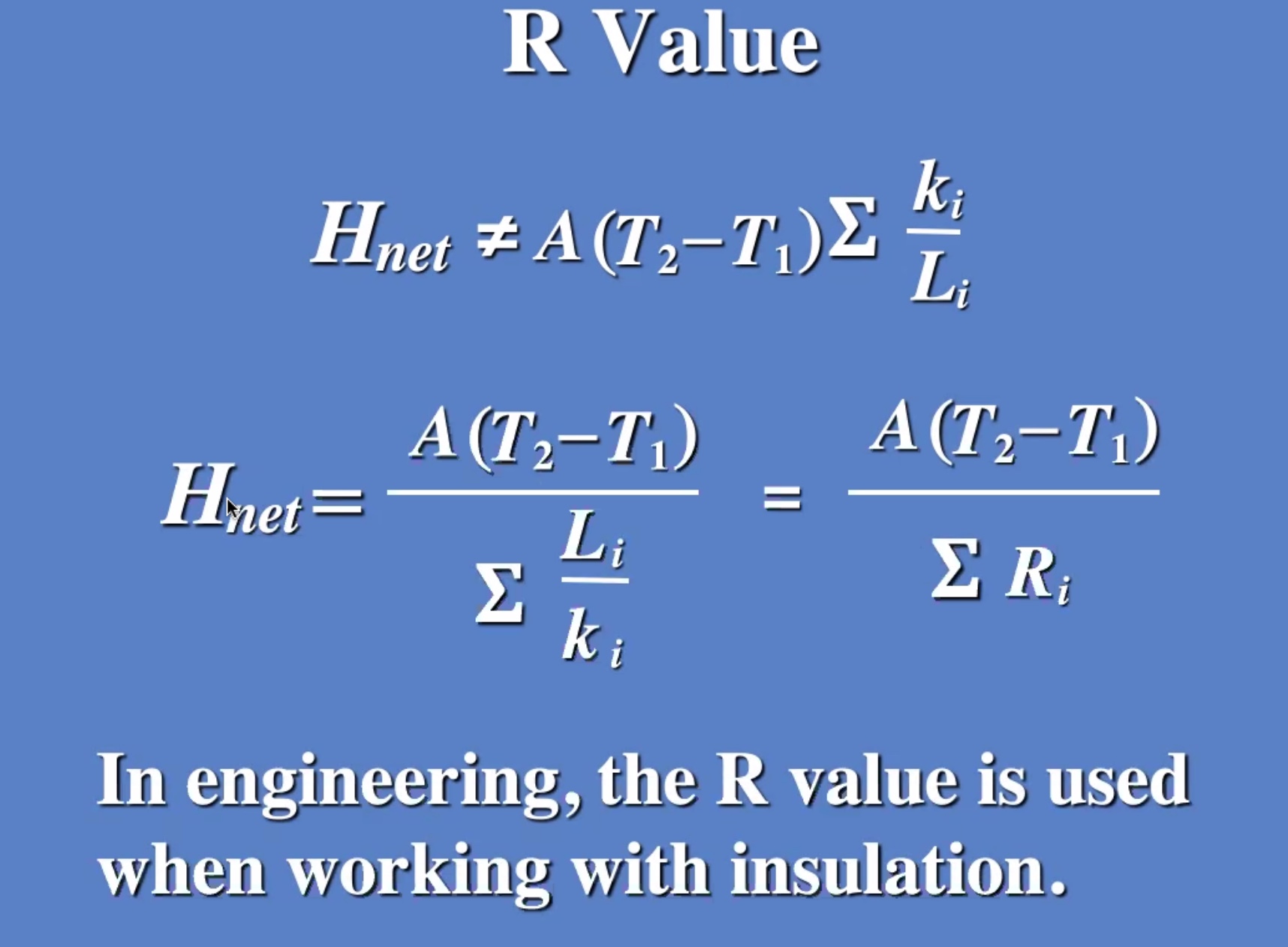
(R) value is like a resistance to heat flow.