Announcements #

Clicker #



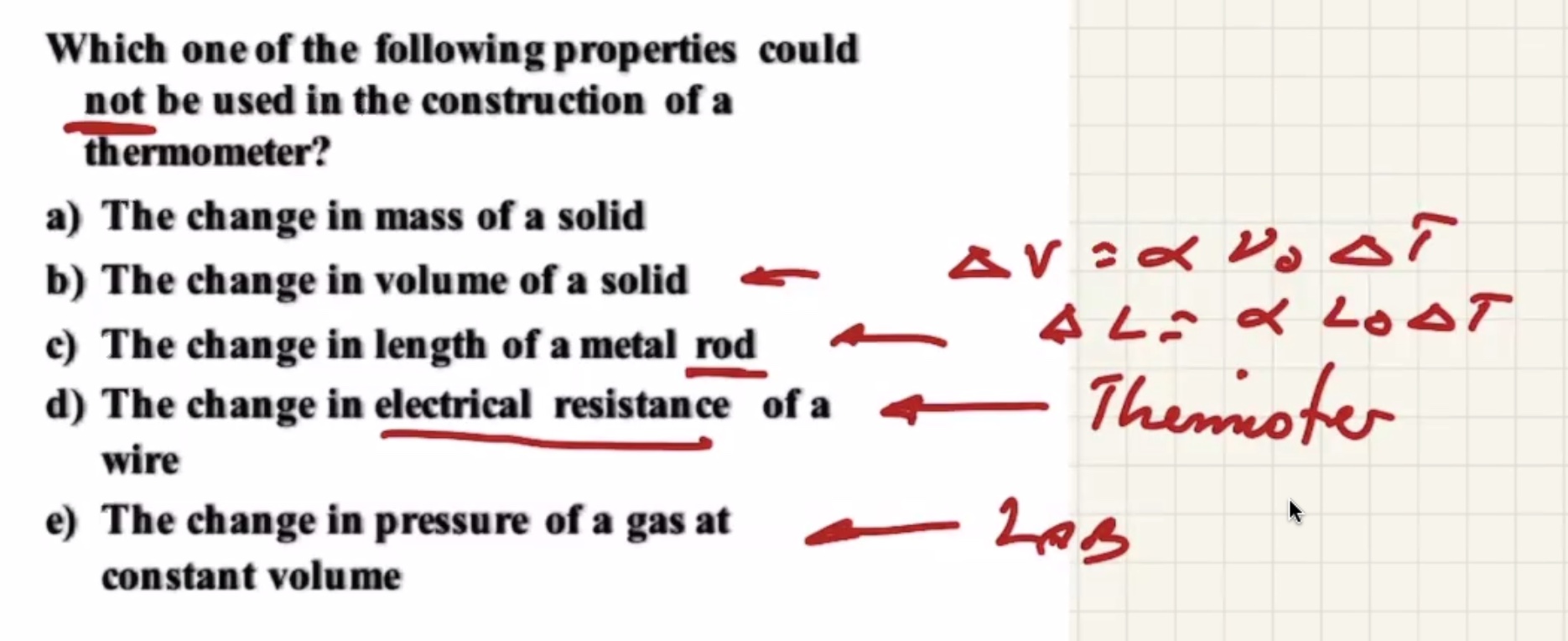
The only thing that doesn’t change with temperature is mass.

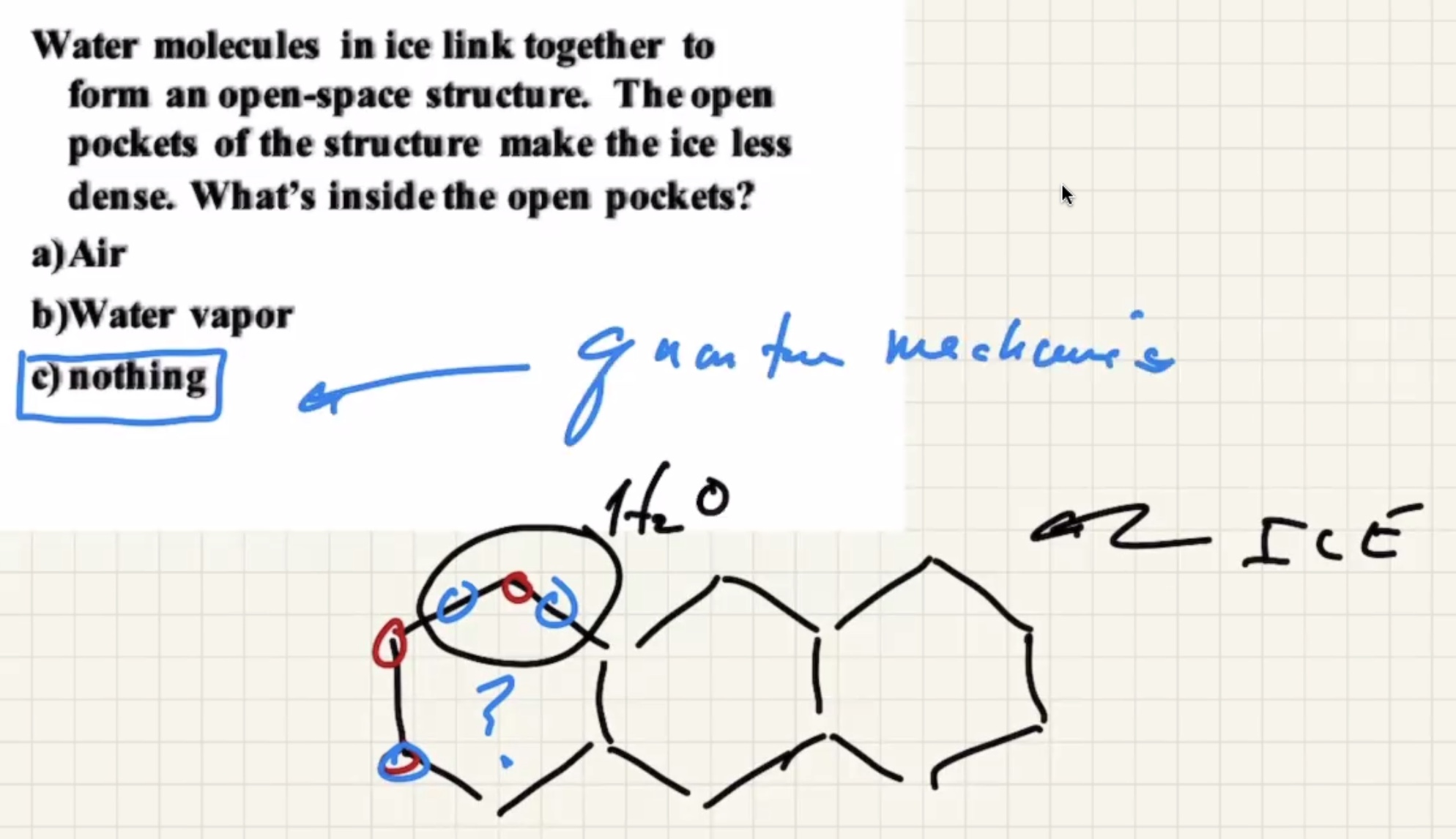
Atoms are also mostly empty space.
Ideal gas law #
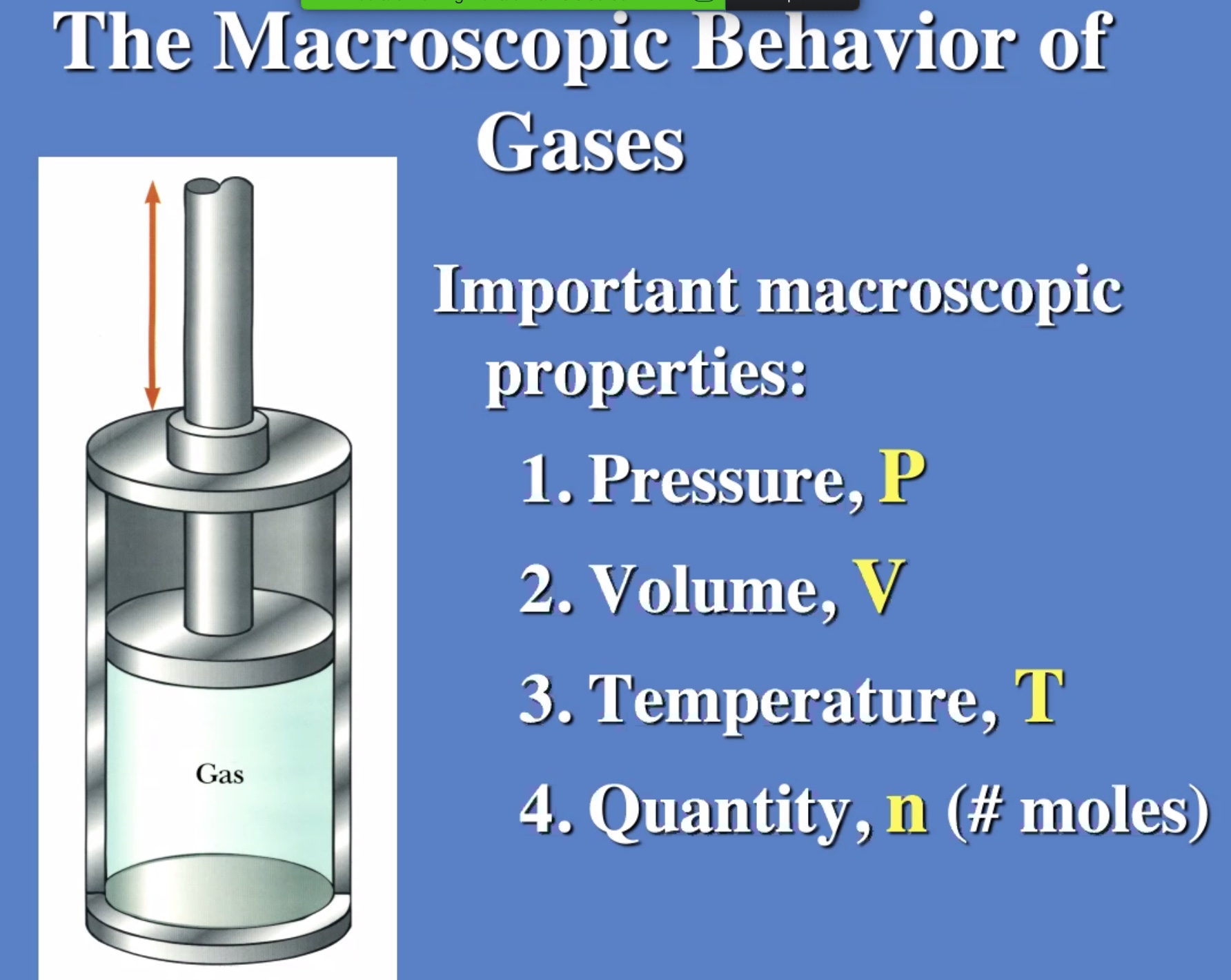
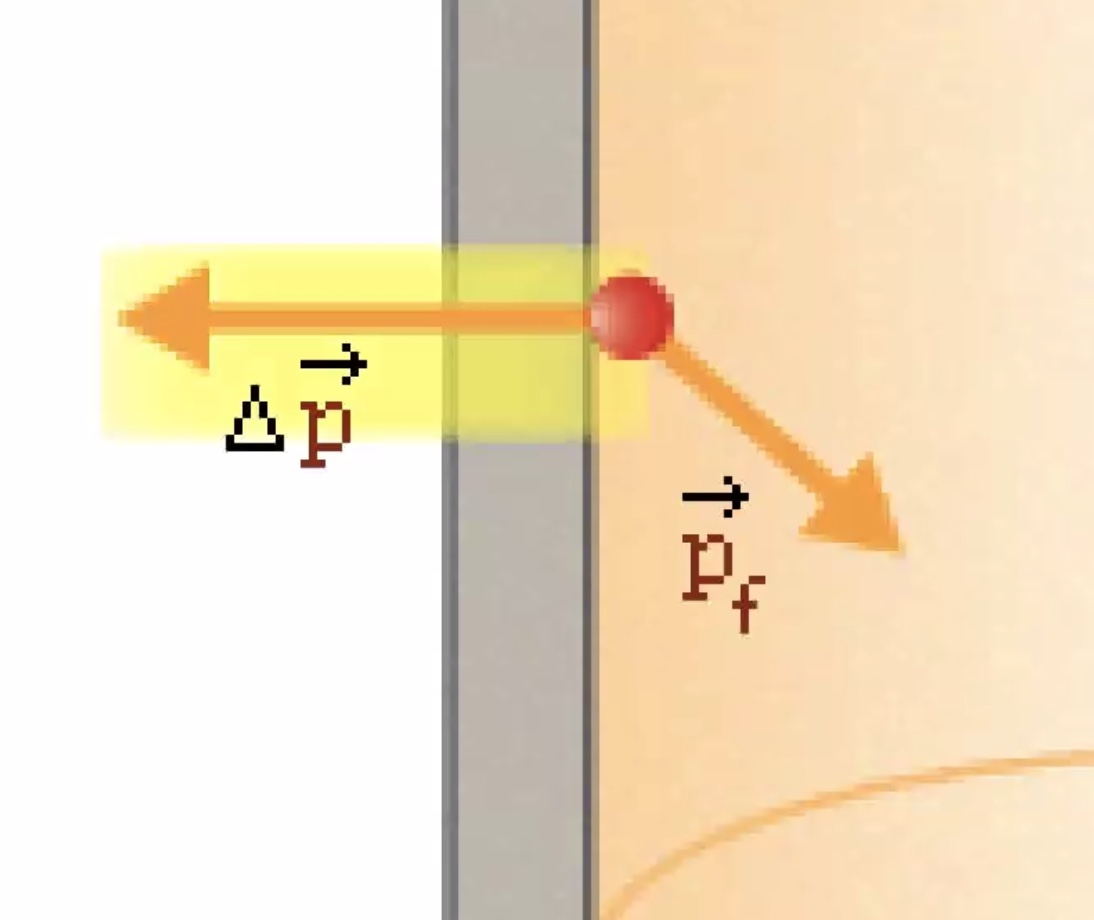
Molecules of a gas bouncing off the walls of a container on average is the gas pressure.
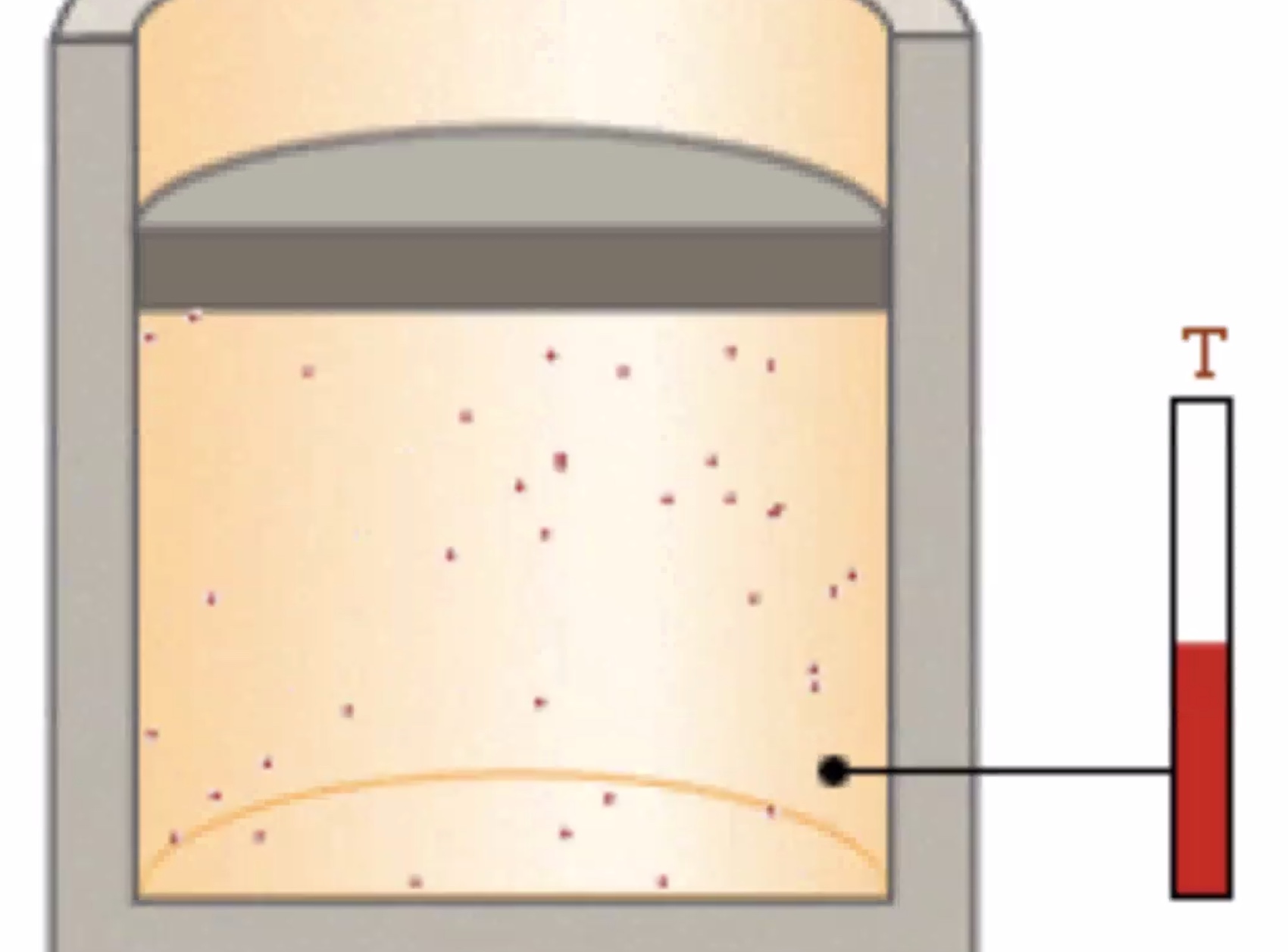
Gas pressure arises from the temperature of the gas molecules.
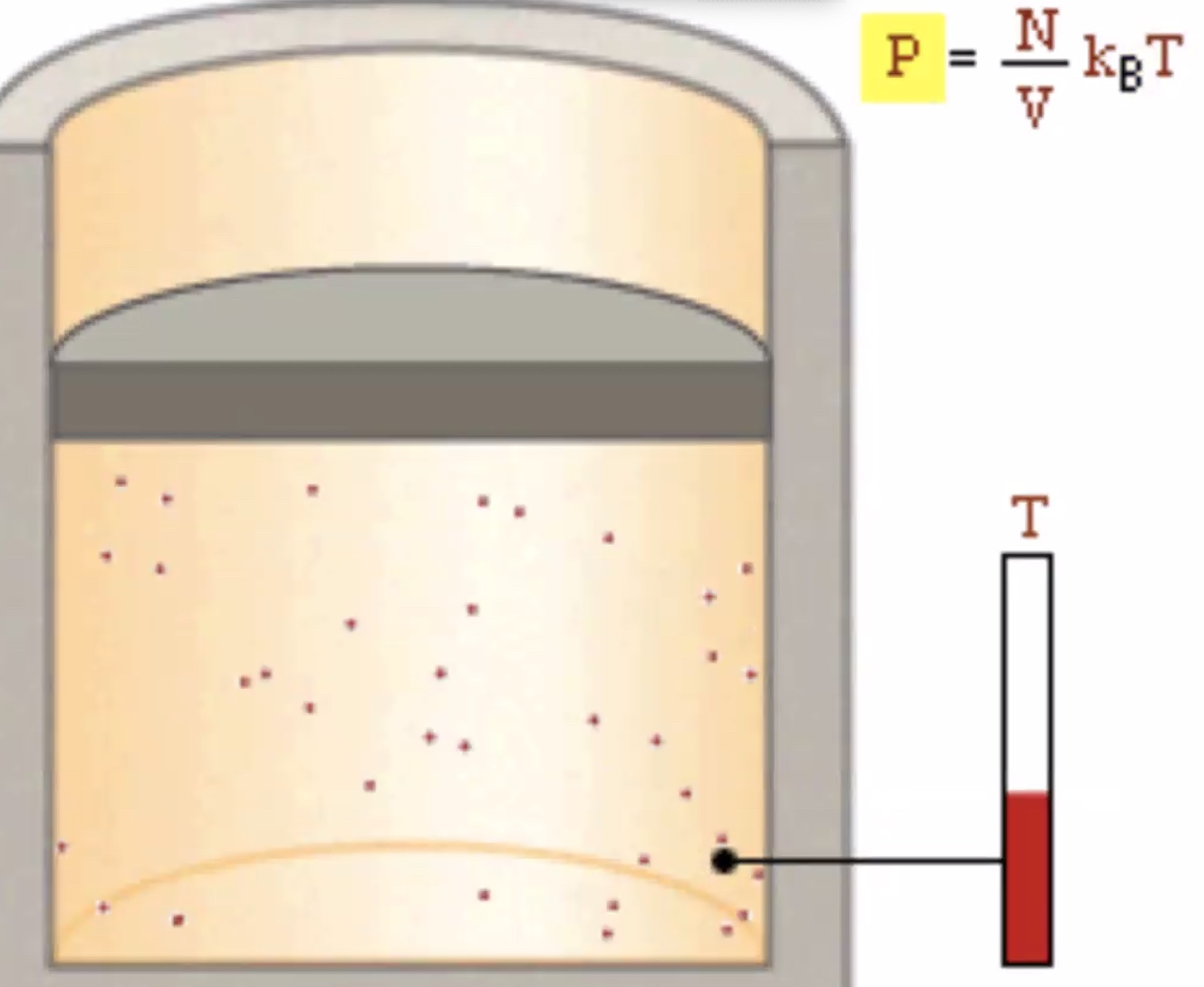
Increasing the temp increases the pressure. Increasing the number of molecules also increases the temperature.
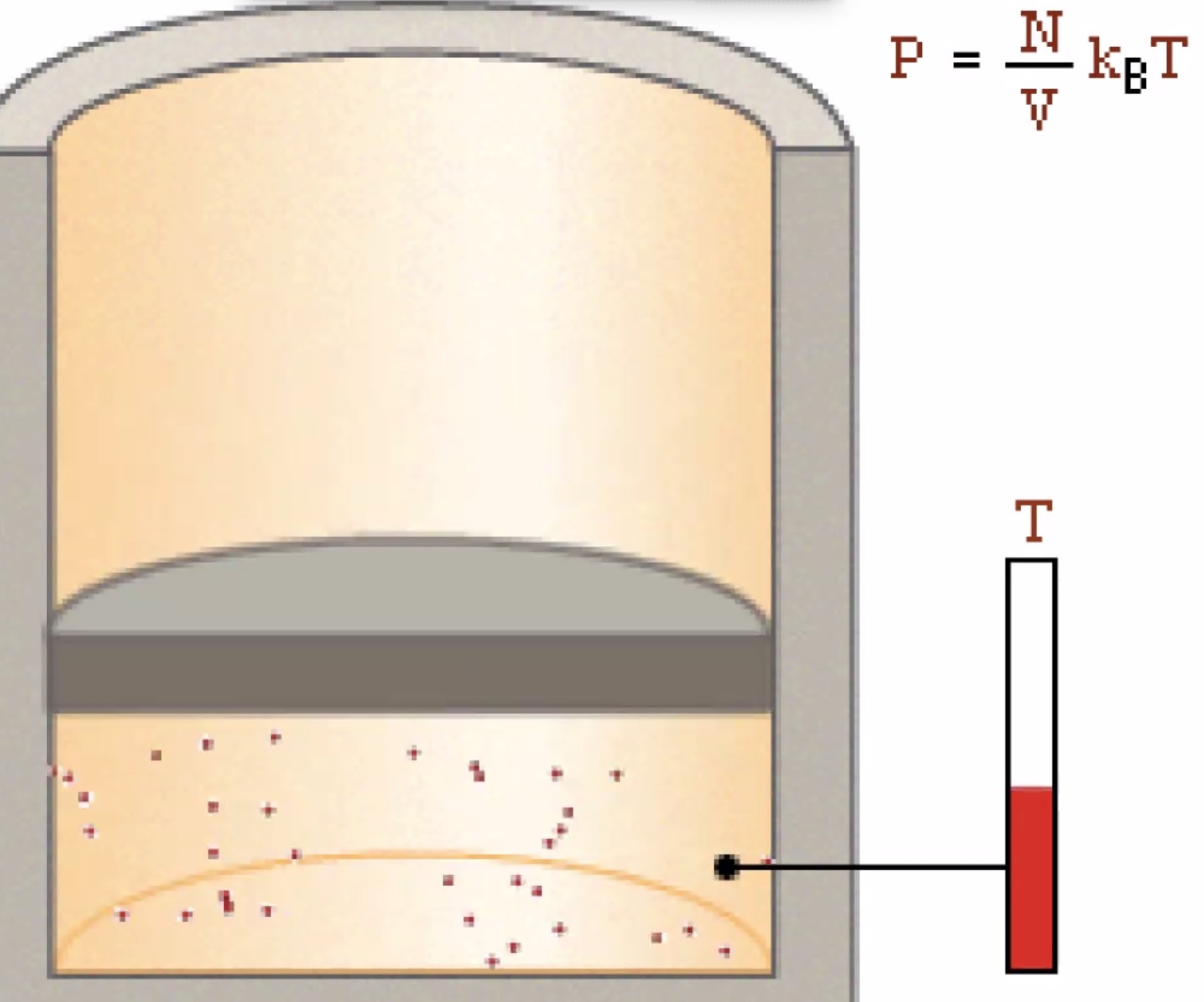
Decreasing the volume of the container increases the pressure.
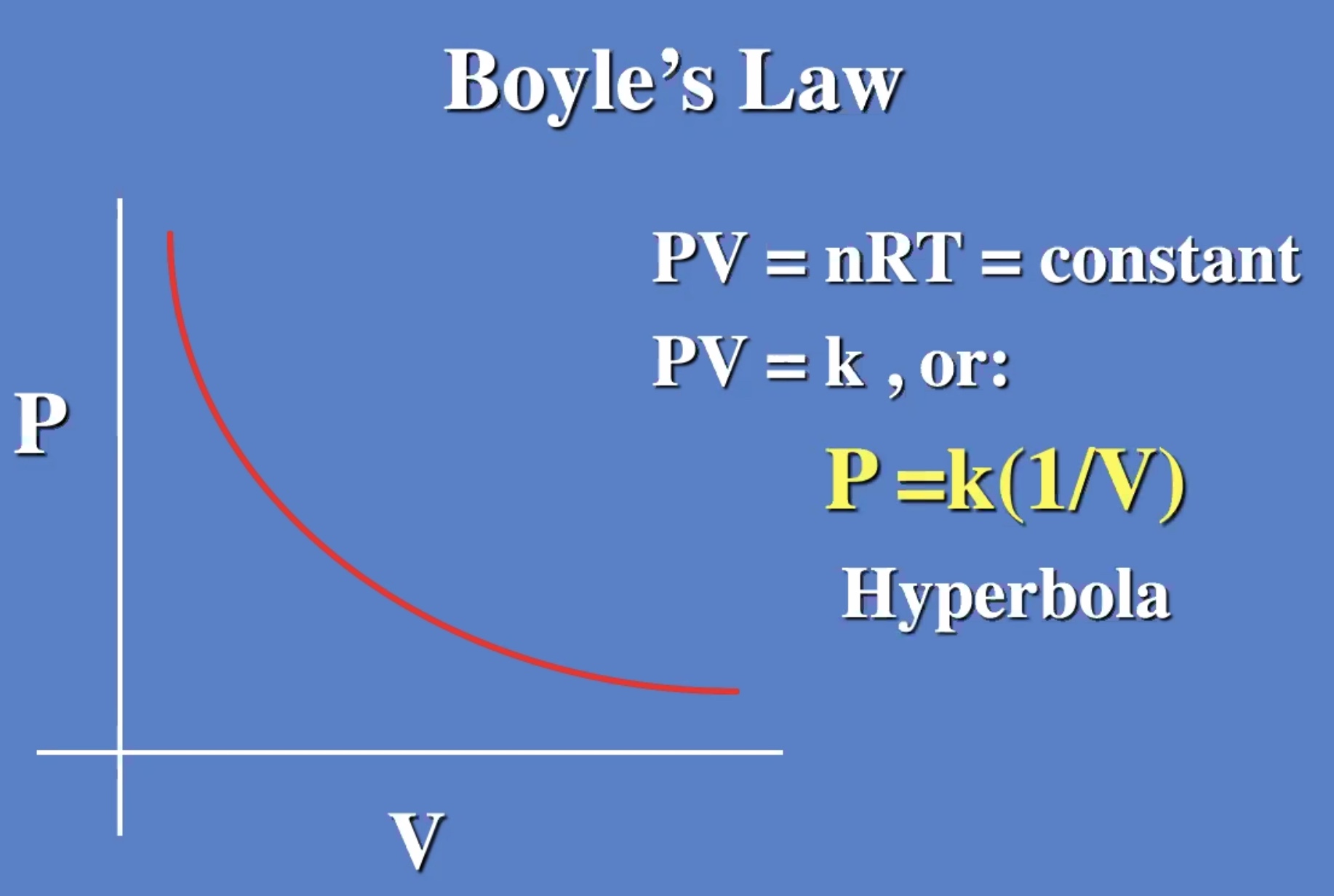
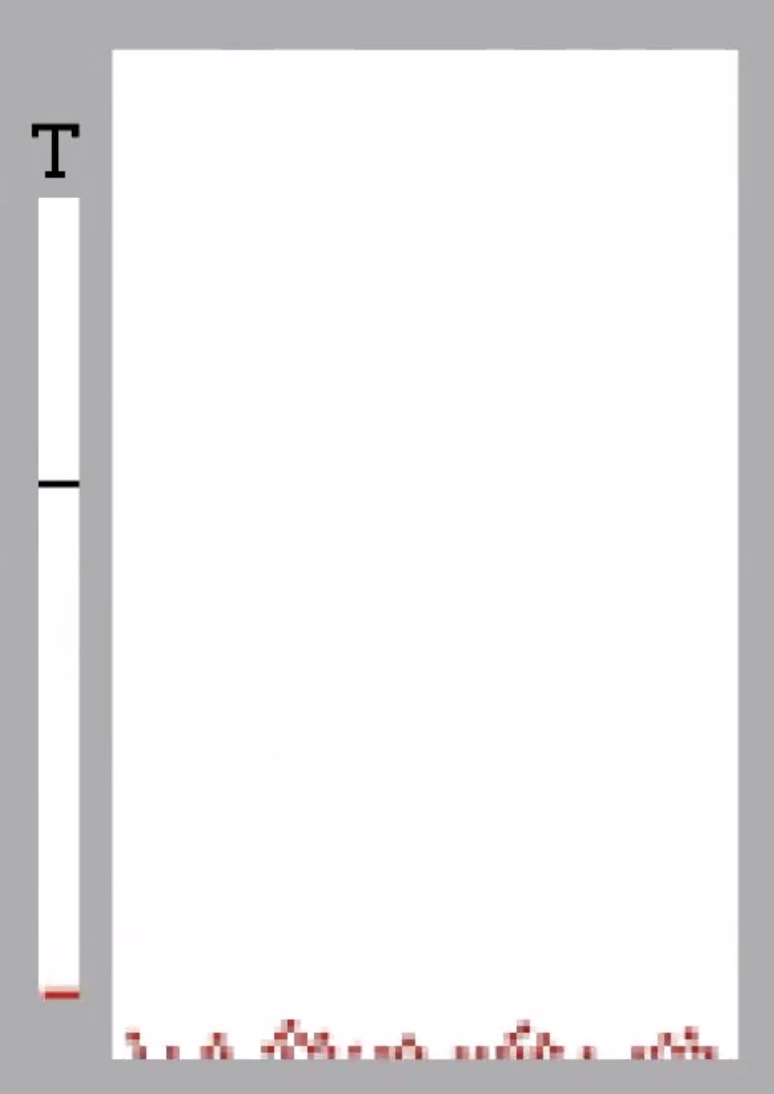
Gases reach their minimum thermal energy at 0 kelvin (absolute zero). Because of quantum mechanics, you can never stop all molecular motion.
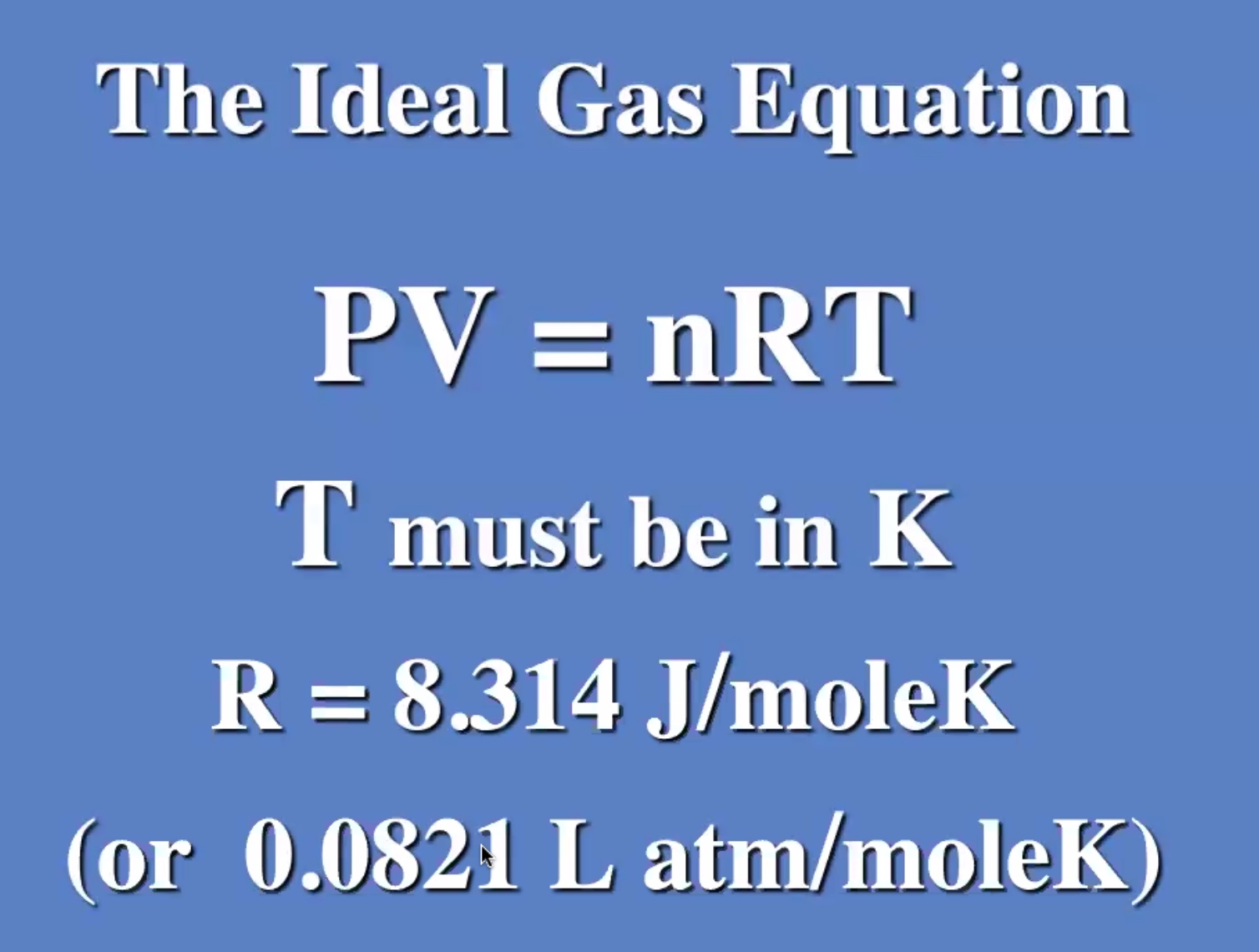
This was understood before even the molecular theory.

Examples #

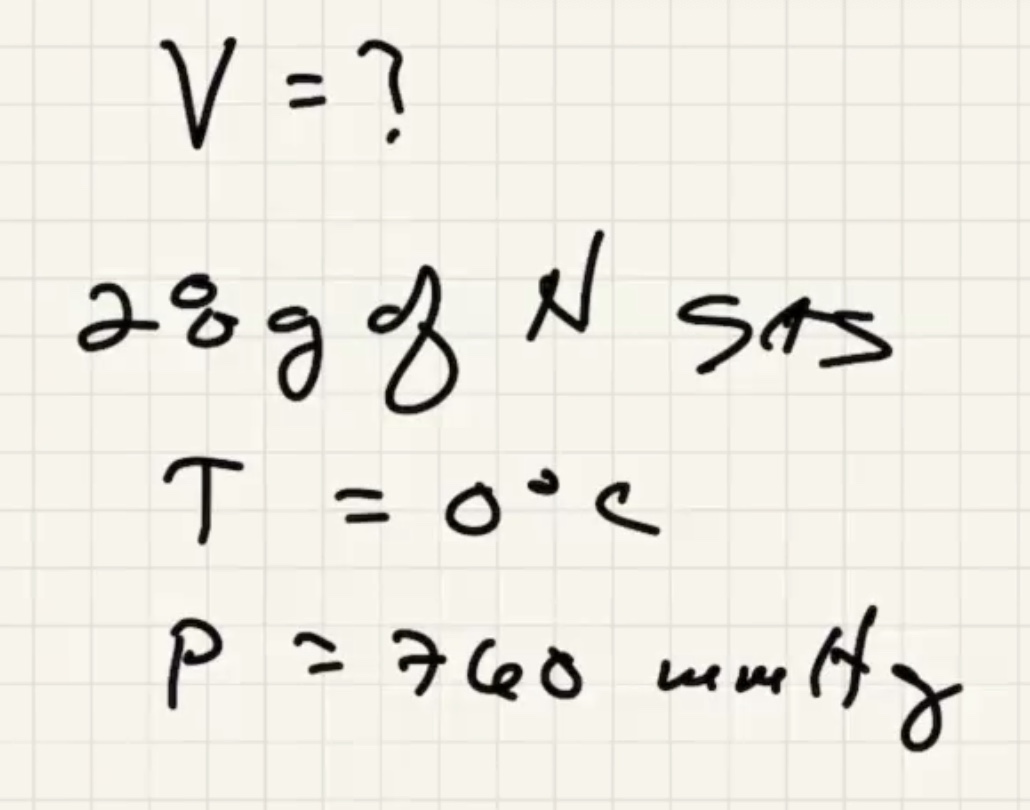
Lets use the ideal gas law:
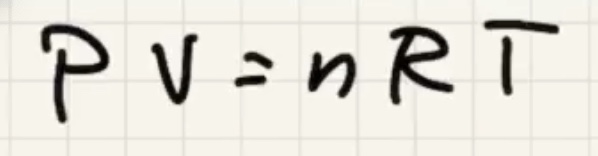
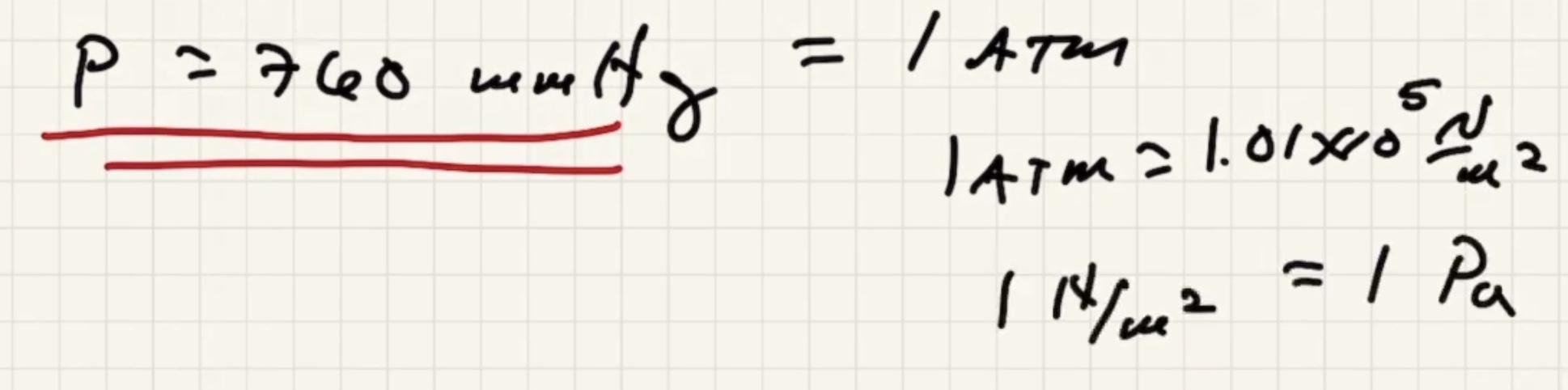

So lets interpret this:
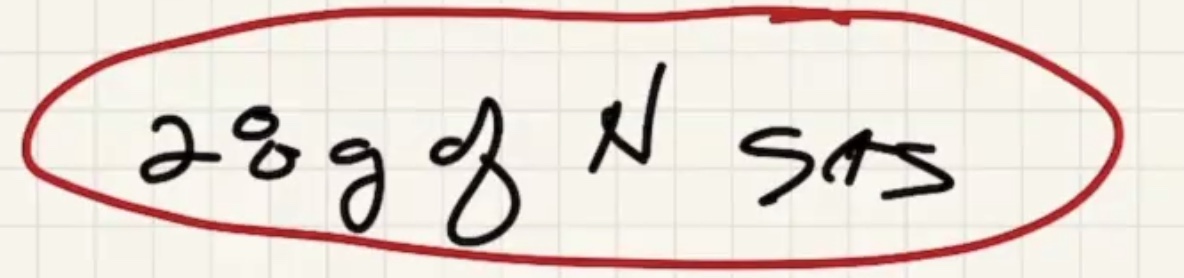
Check the periodic table for the atomic number of N (the number of protons/electrons in an atom). And the bottom number is the atomic mass in g/mol
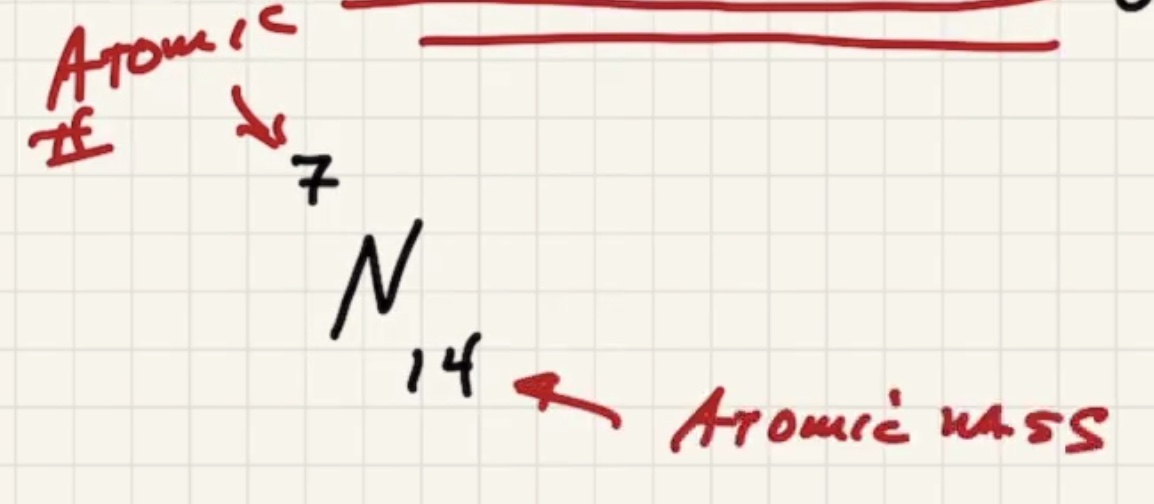
Remember that nitrogen in the gasous state is a diatomic molecule. Since there are two molecules, it has 26 grams per mole.
Solve for the volume:
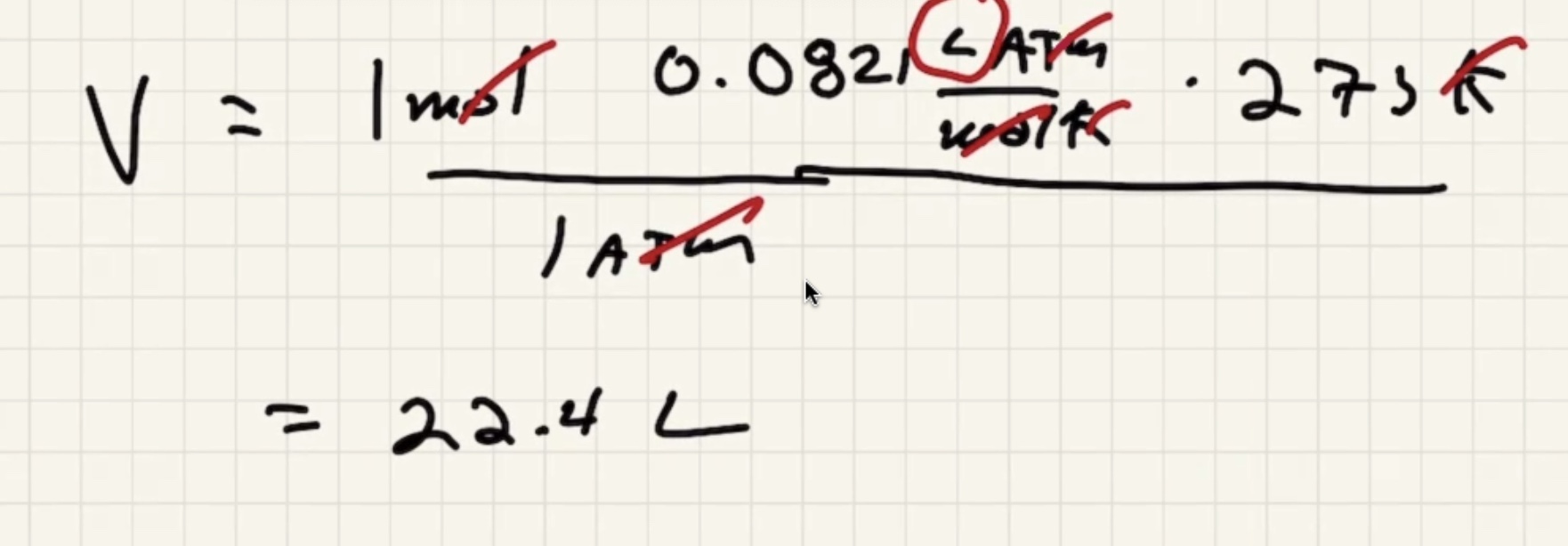
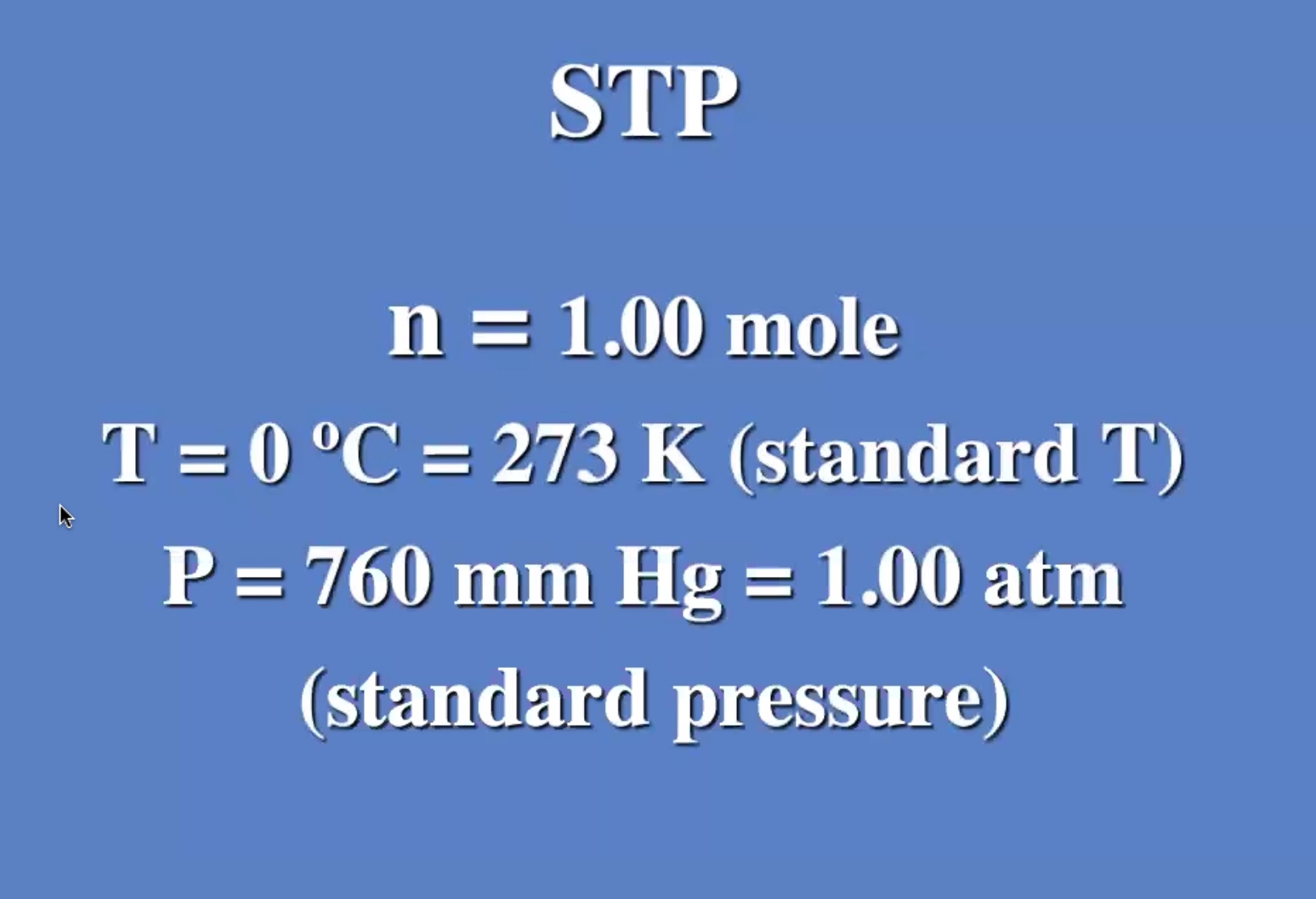
All gases at standard temp and pressure, all gases occupy the same volume regardless of the makeup.



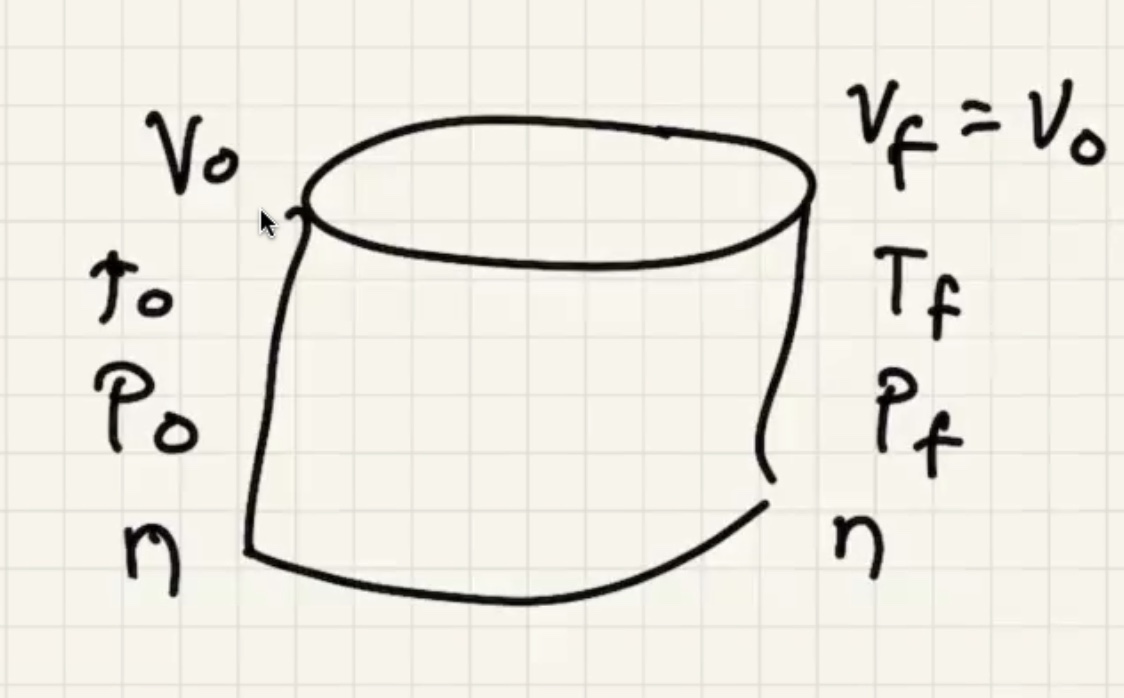
Start with the ideal gas law:
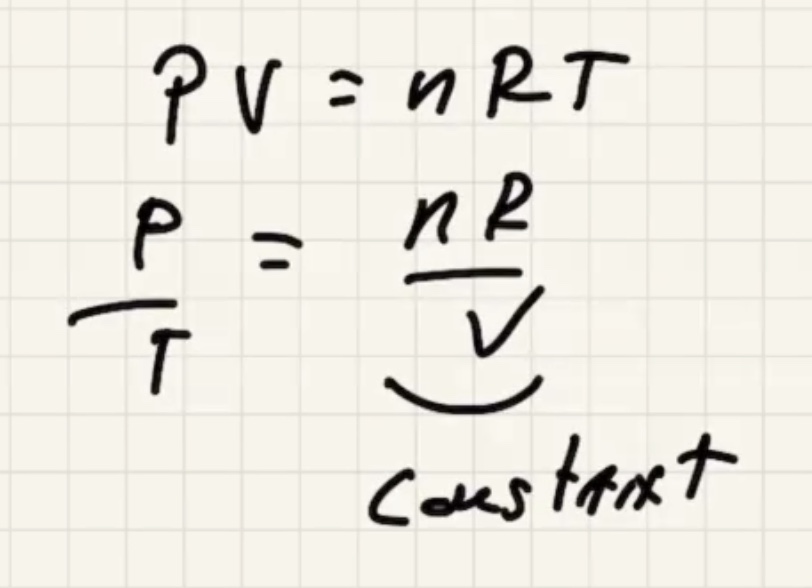
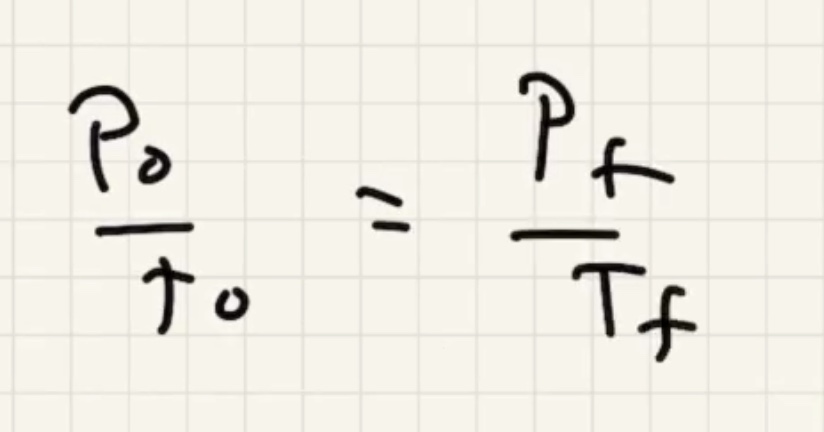
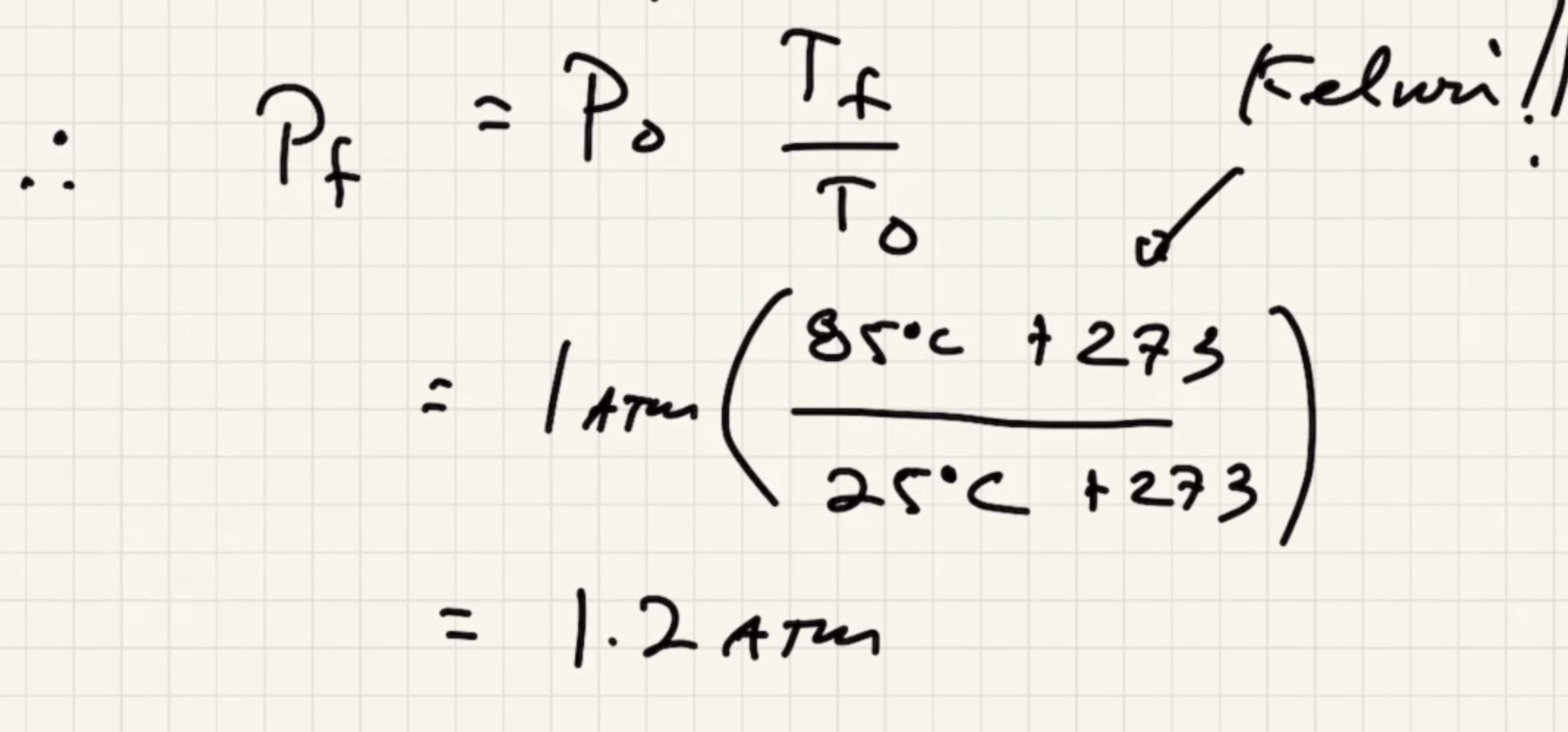
Remember to convert to kelvin to use the ideal gas law.

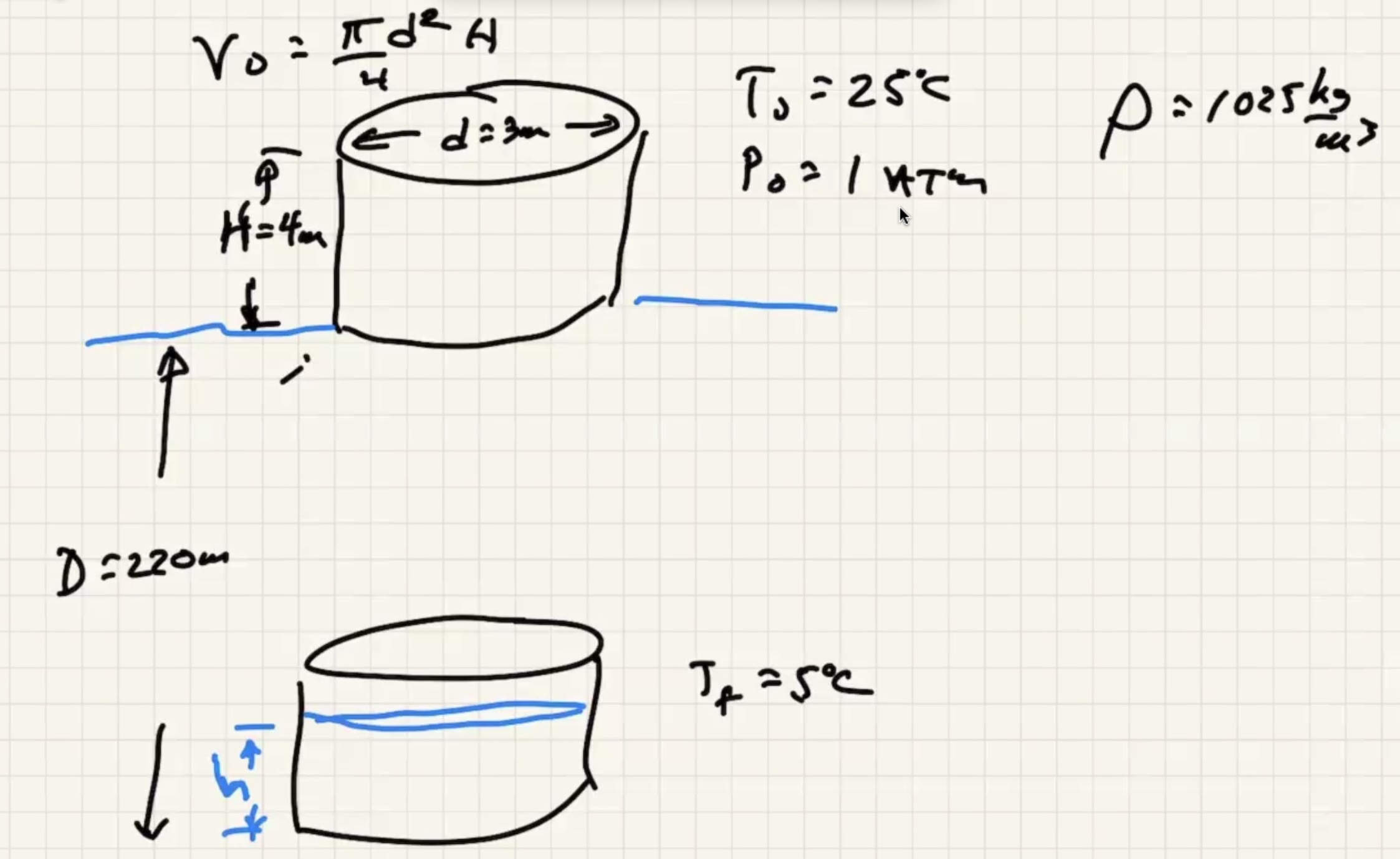
We’re looking for (h) here where the red portion is the gas in the bell:
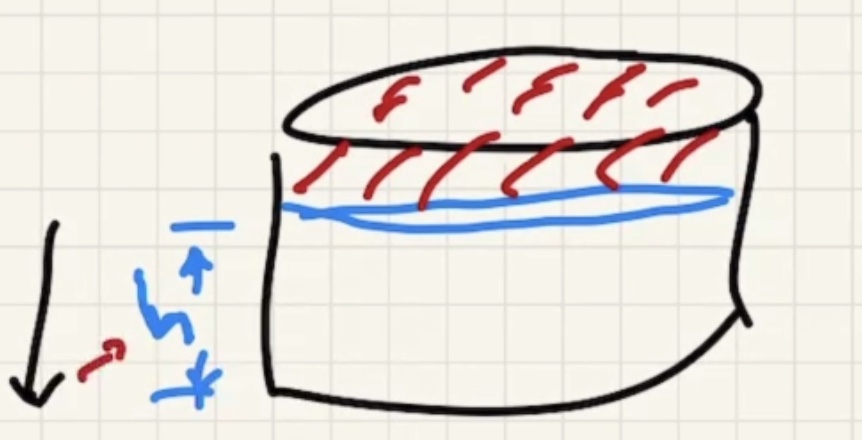
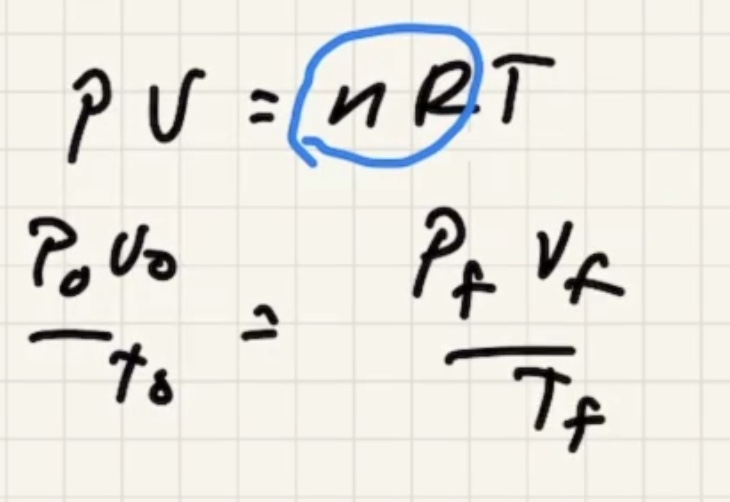
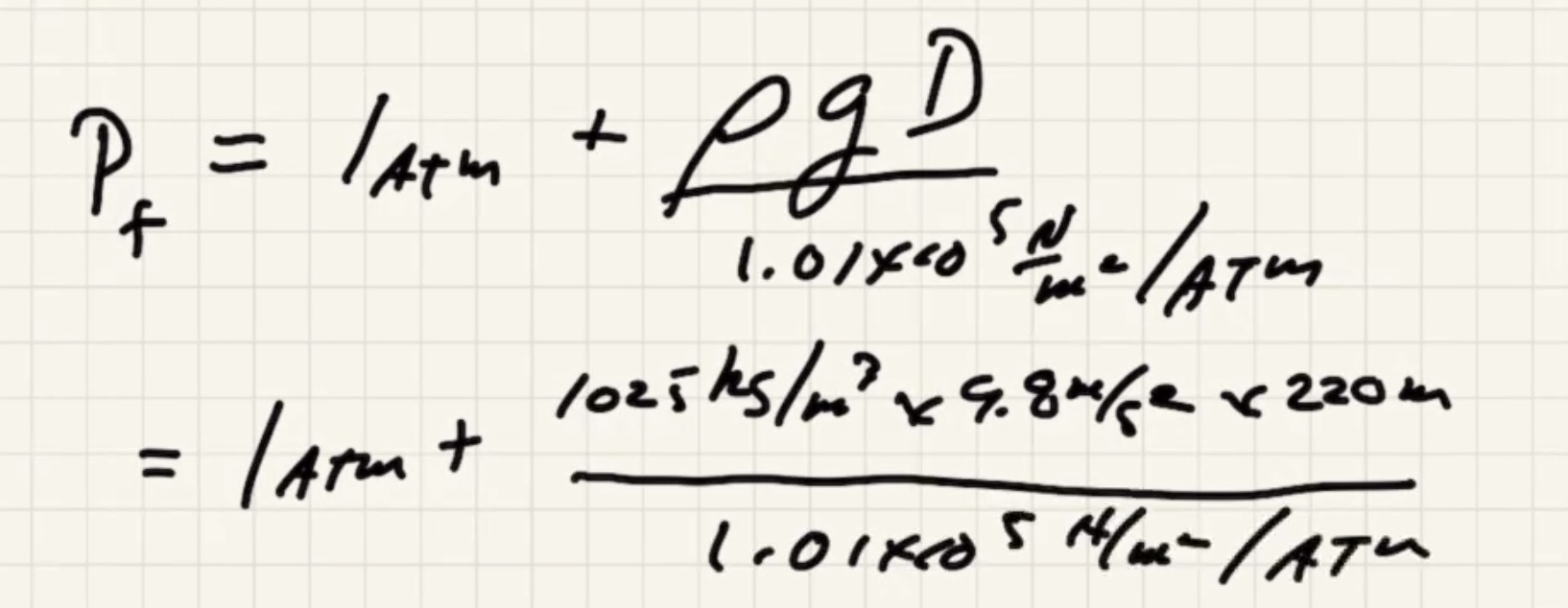
We need to find the pressure final (P_f).
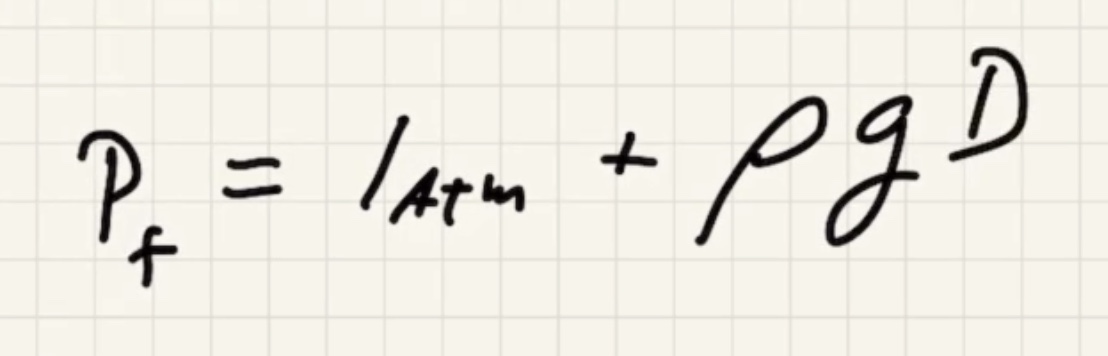
where (D) is the depth of water.
Convert:
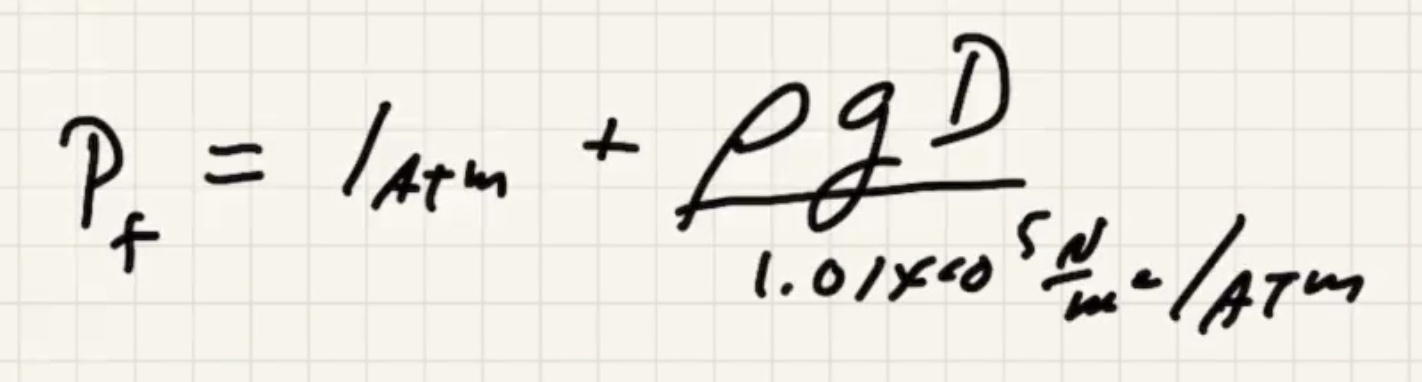
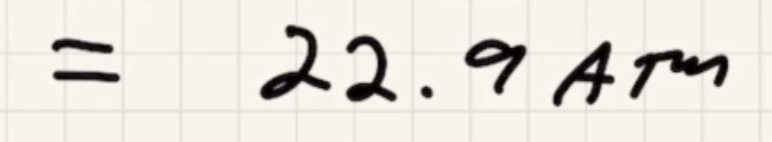
Now we can solve for (v_f)
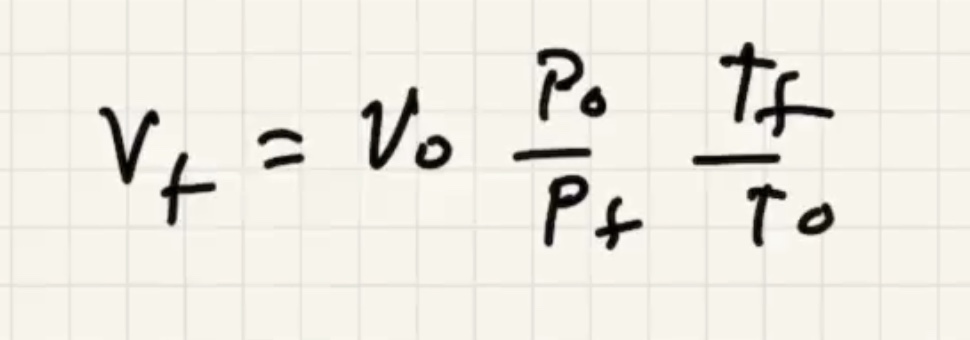
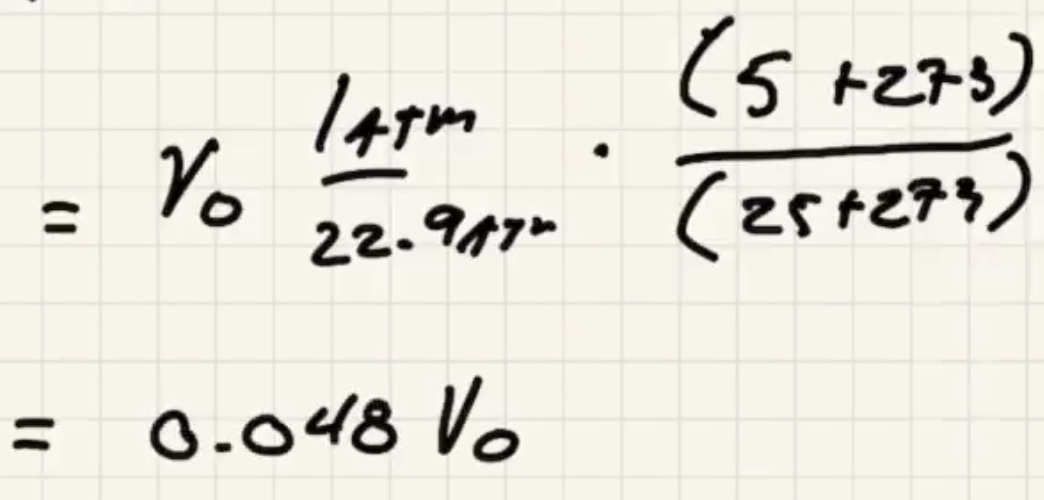
Lets find out what (v_o) is so we can finish it.
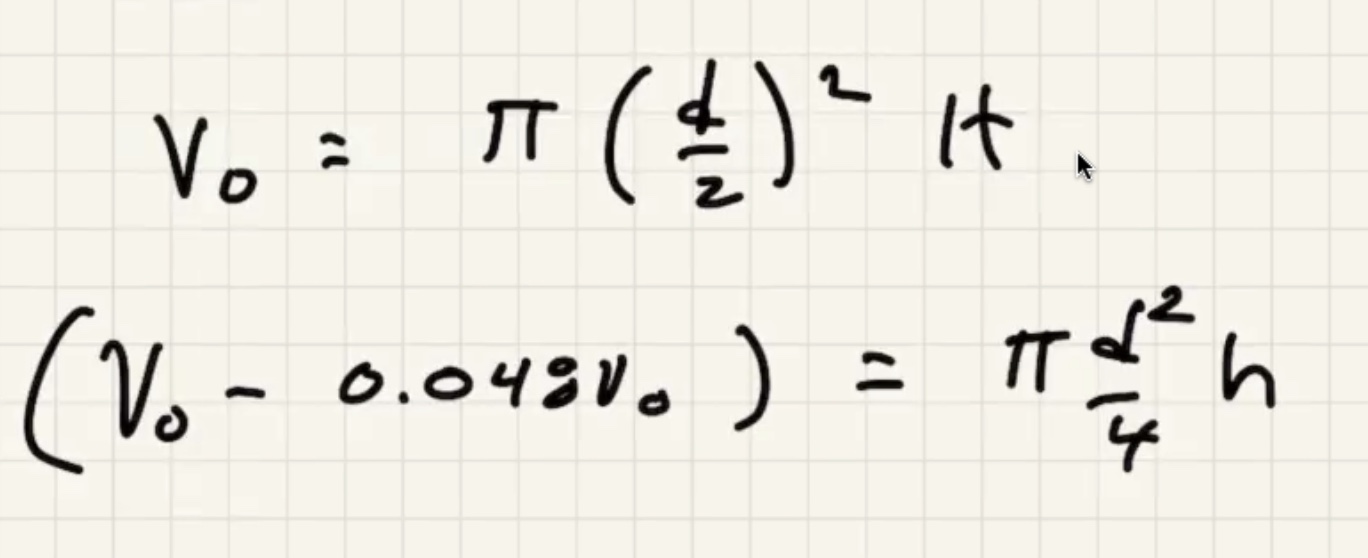


This problem has some similarities to the next quiz.