Announcements #

Today #

Clickers #


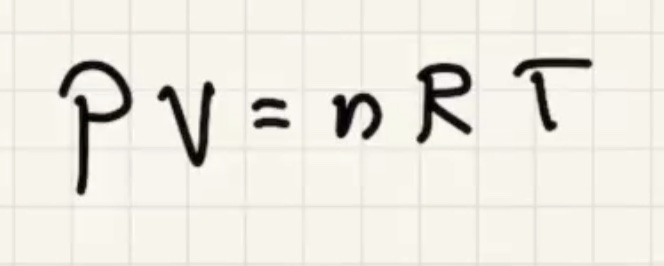
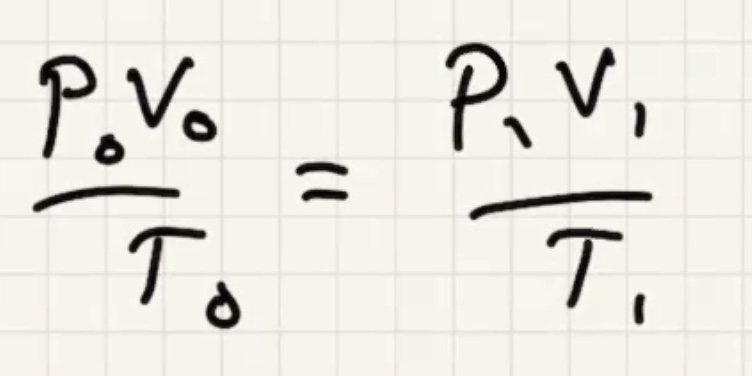
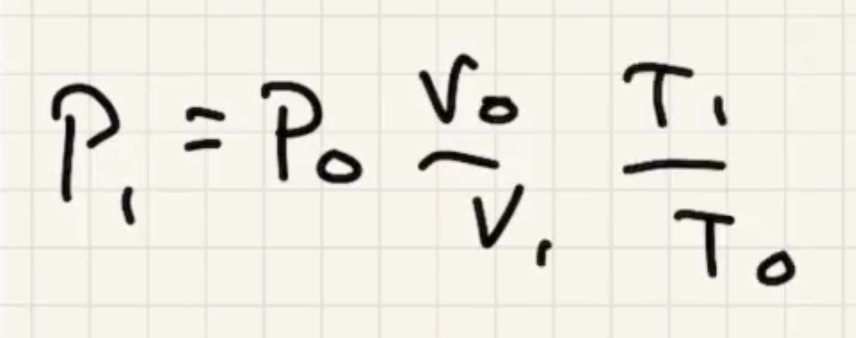
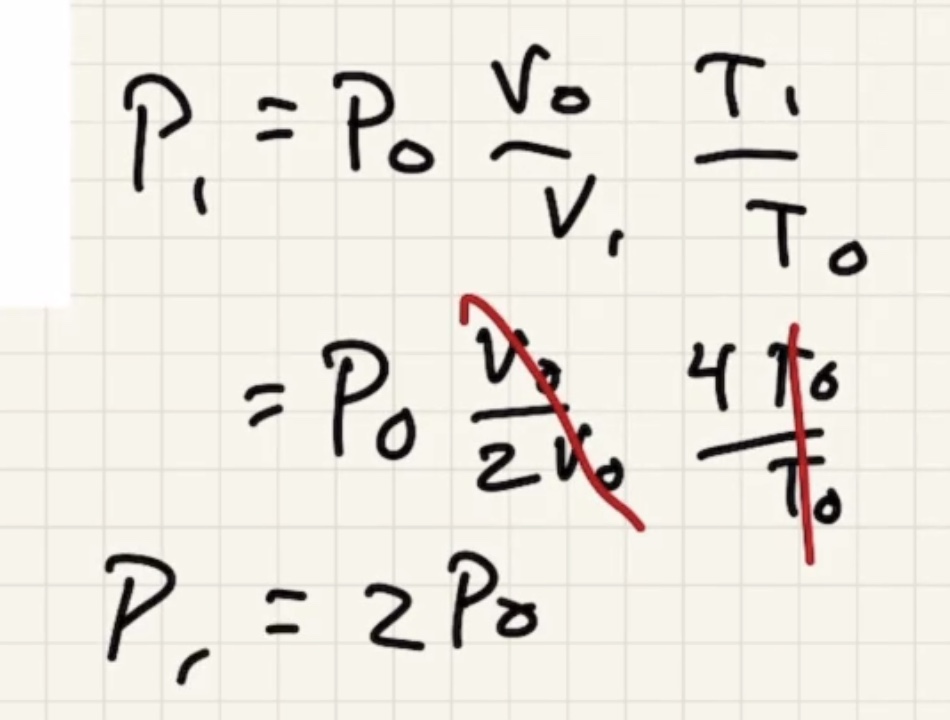


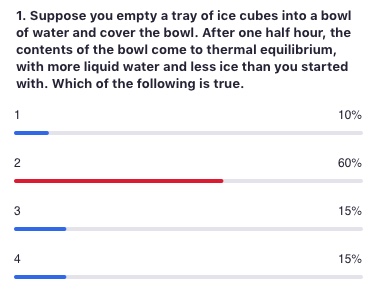
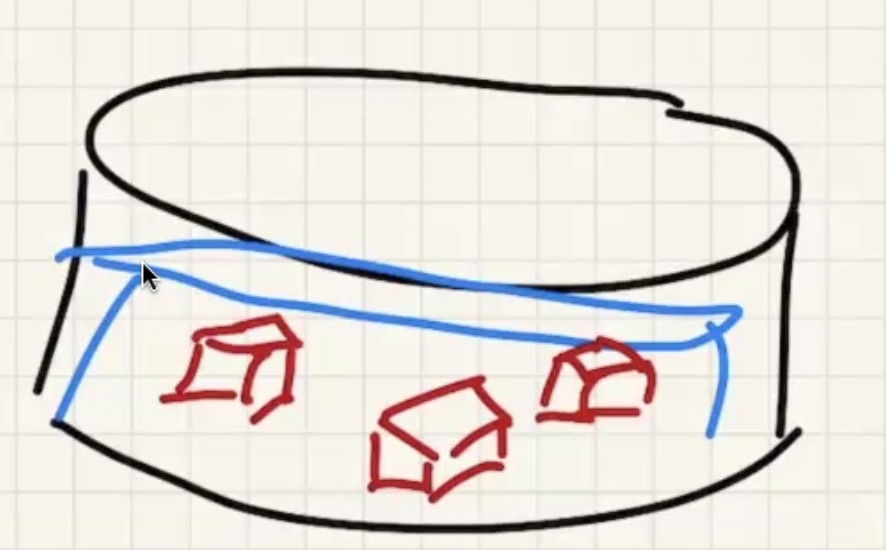
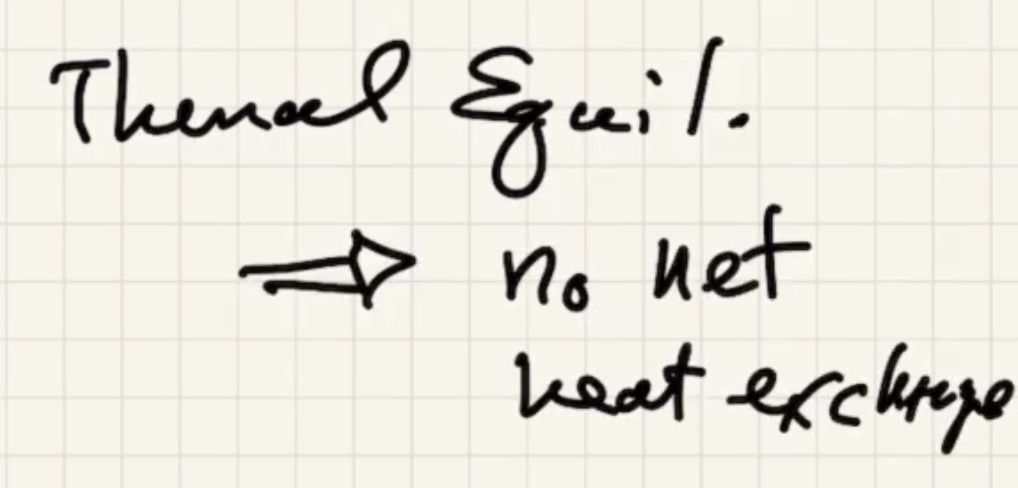
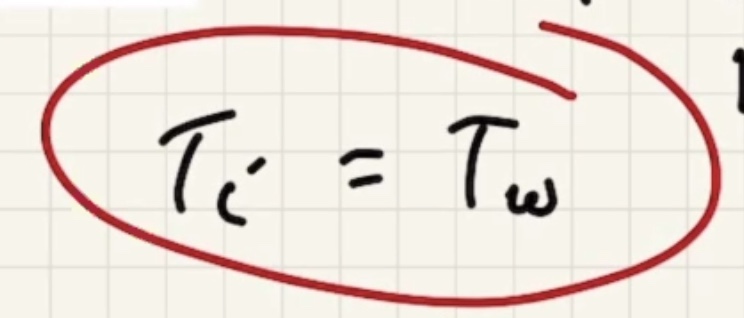
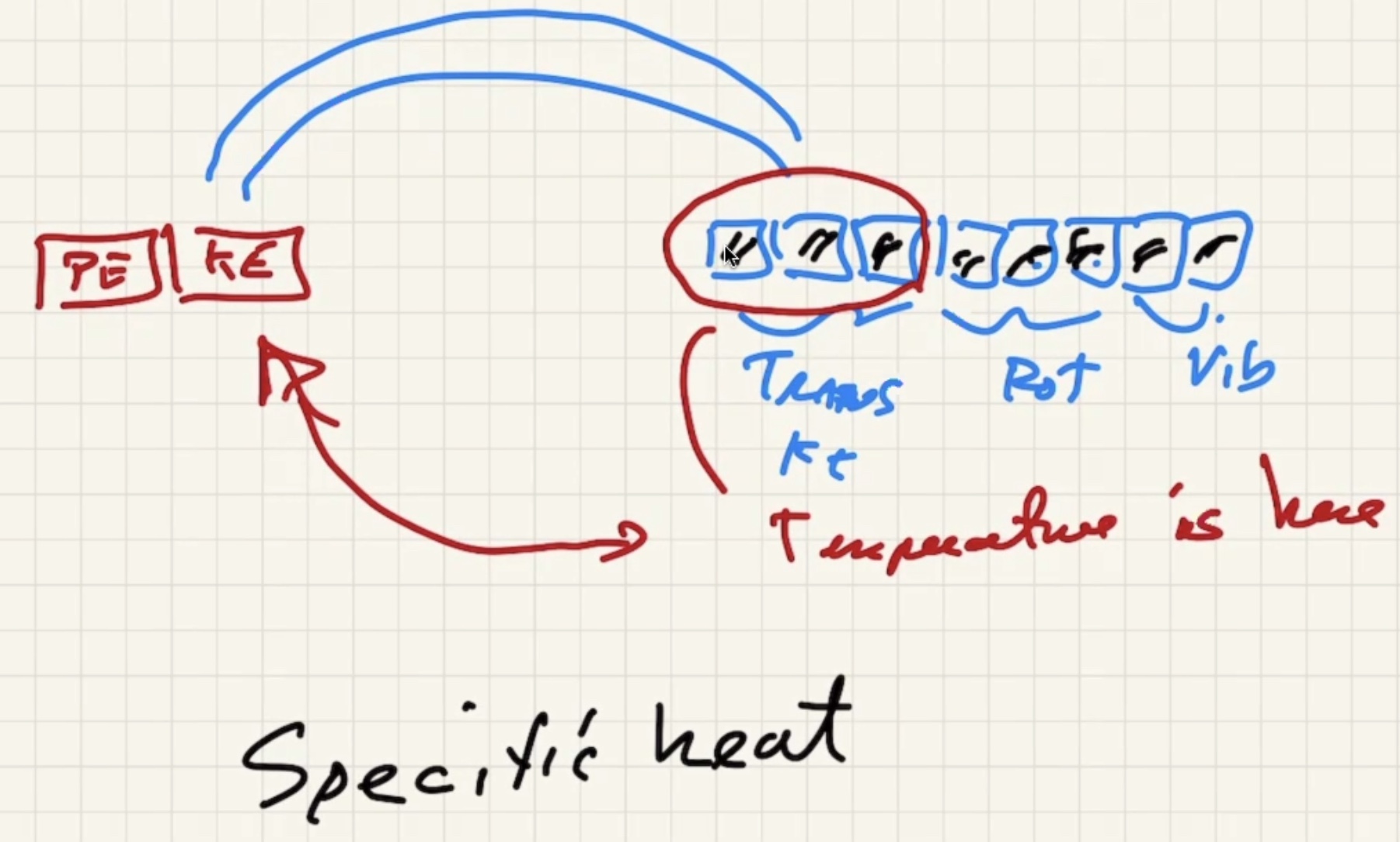
Remember temperature is the average kinetic energy of the system.
Discussion #
Note: Ability to derive this next part isn’t included in the test.

Look at a box with one particle:
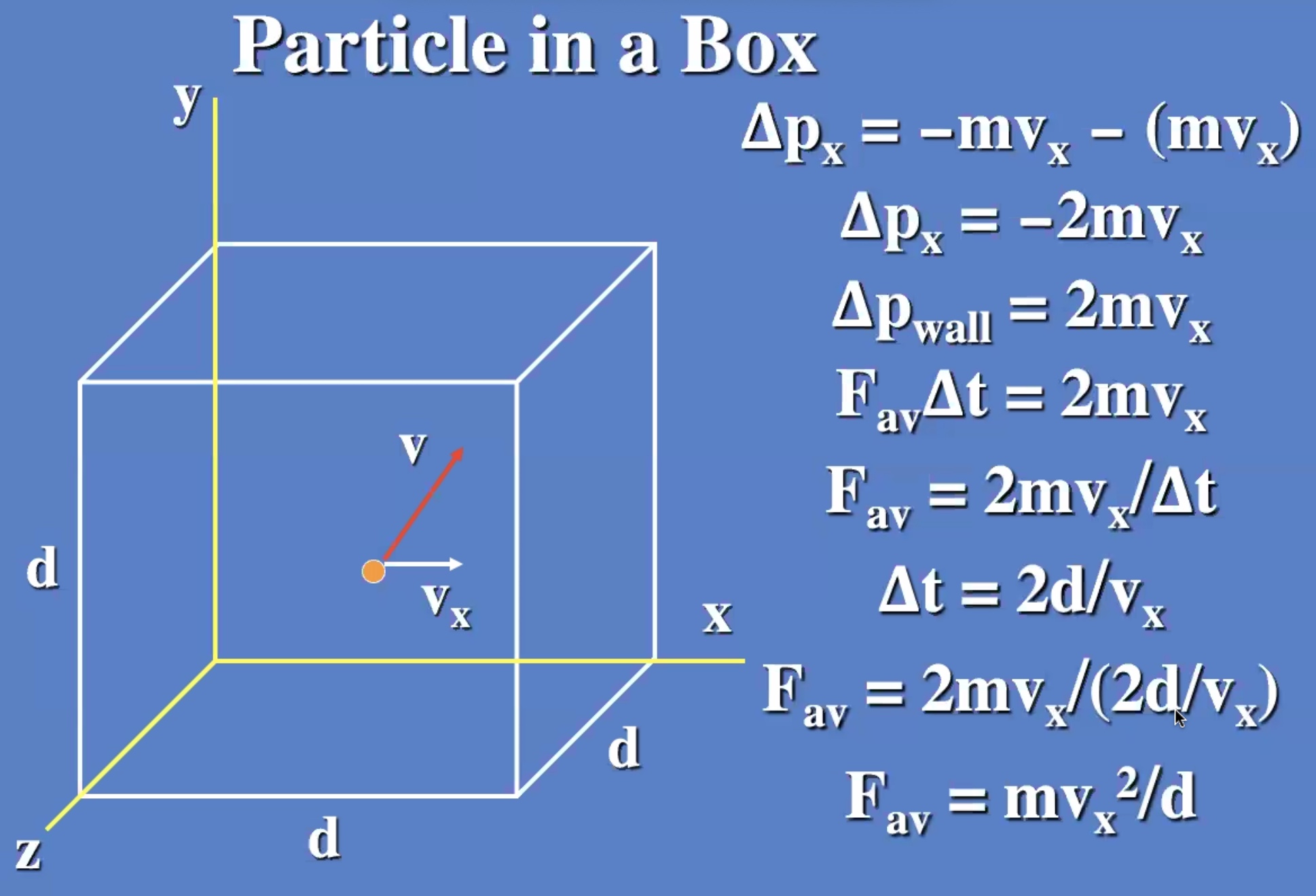
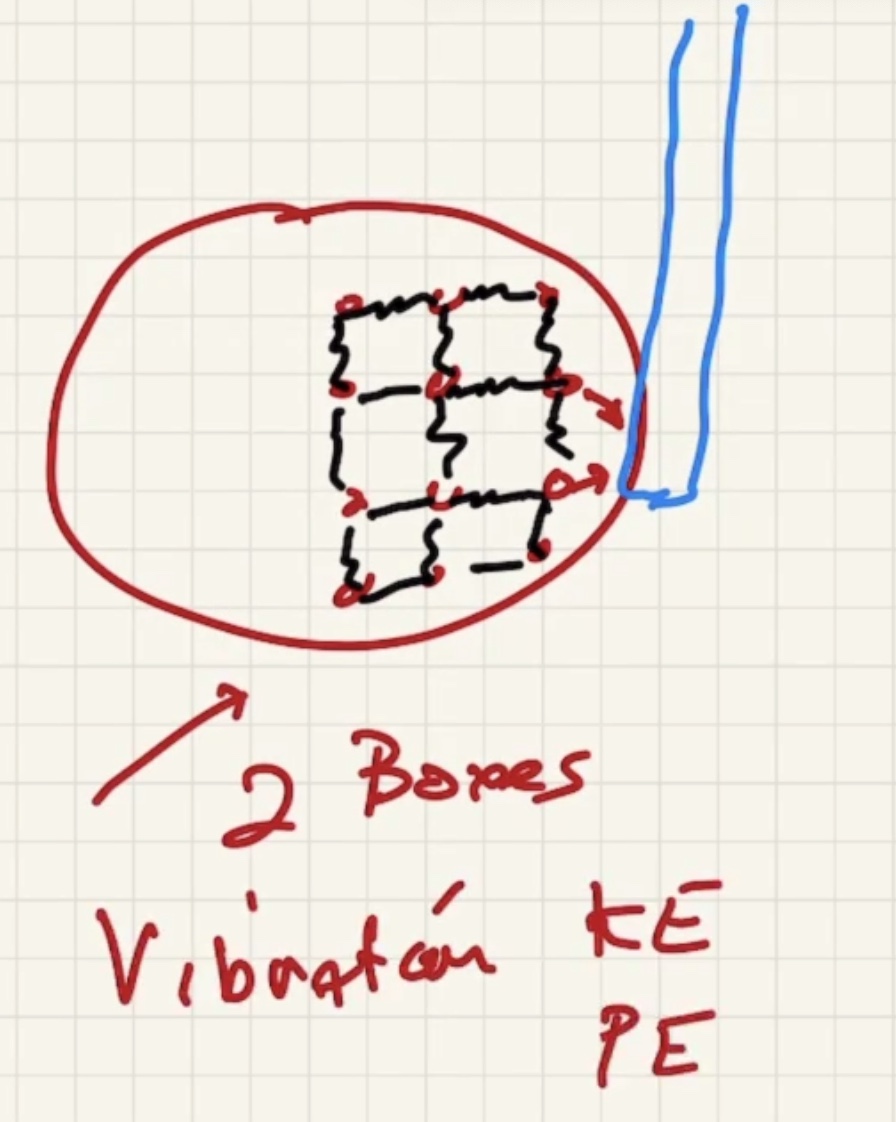
Now lets add a lot more particles:
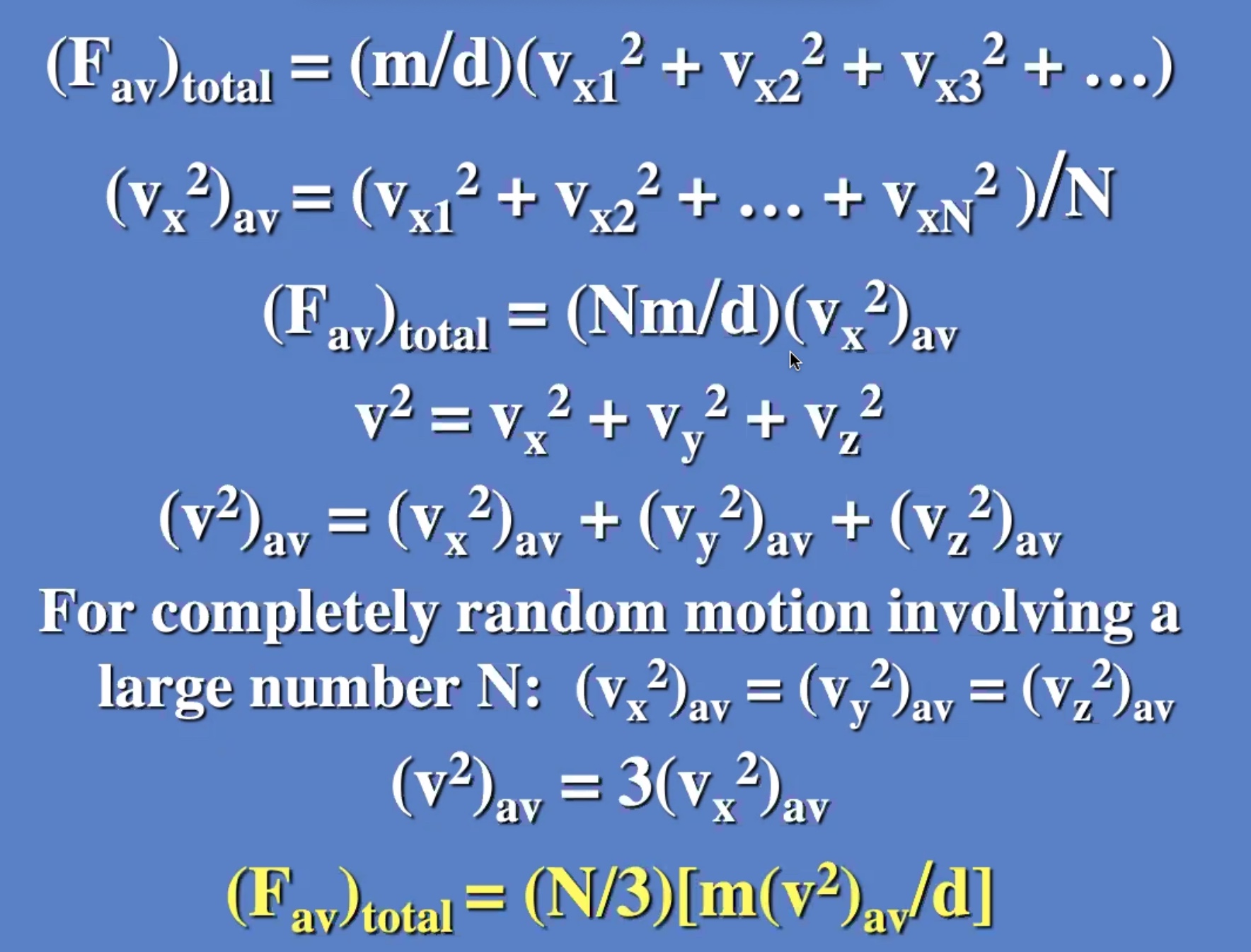


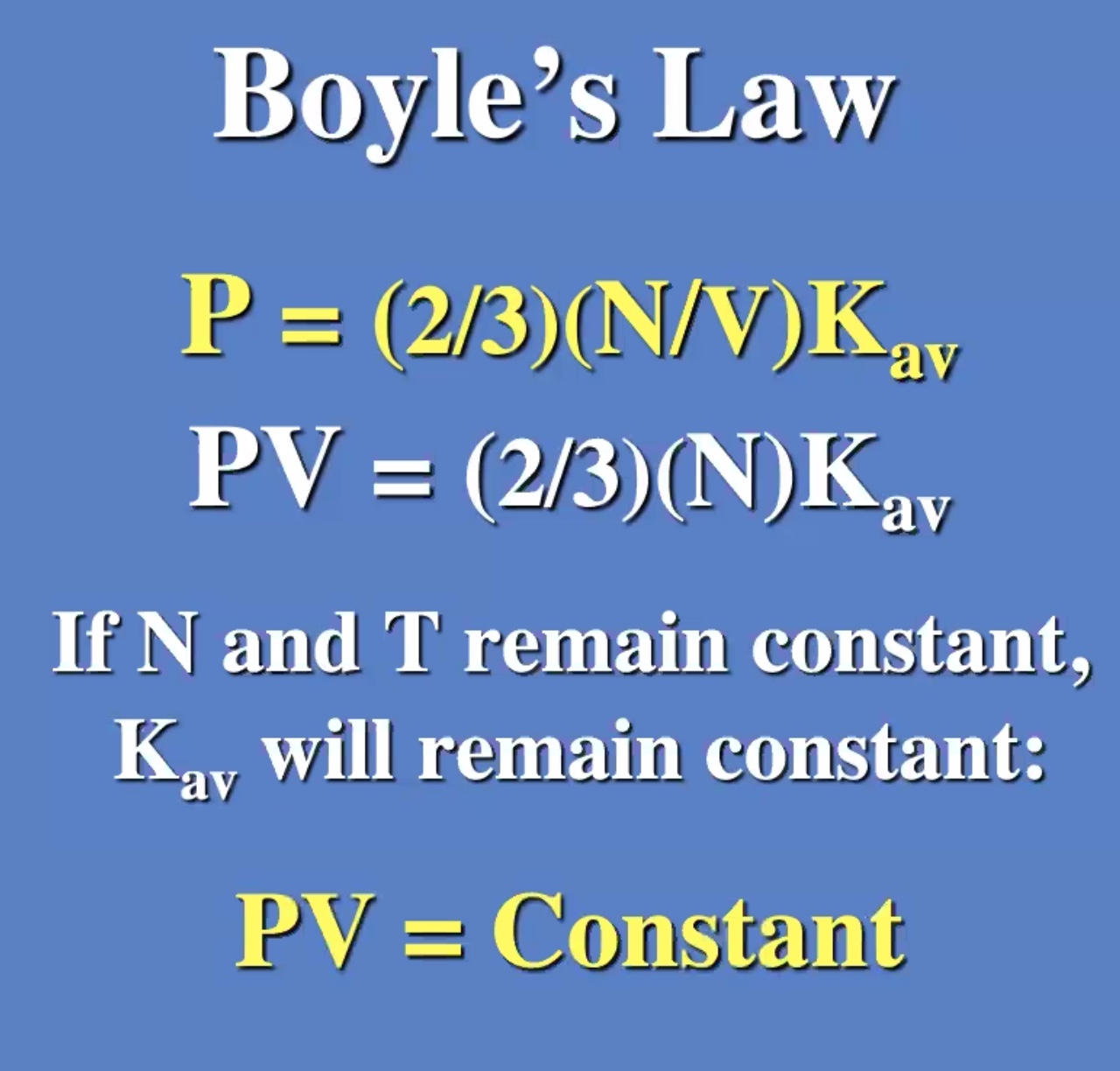
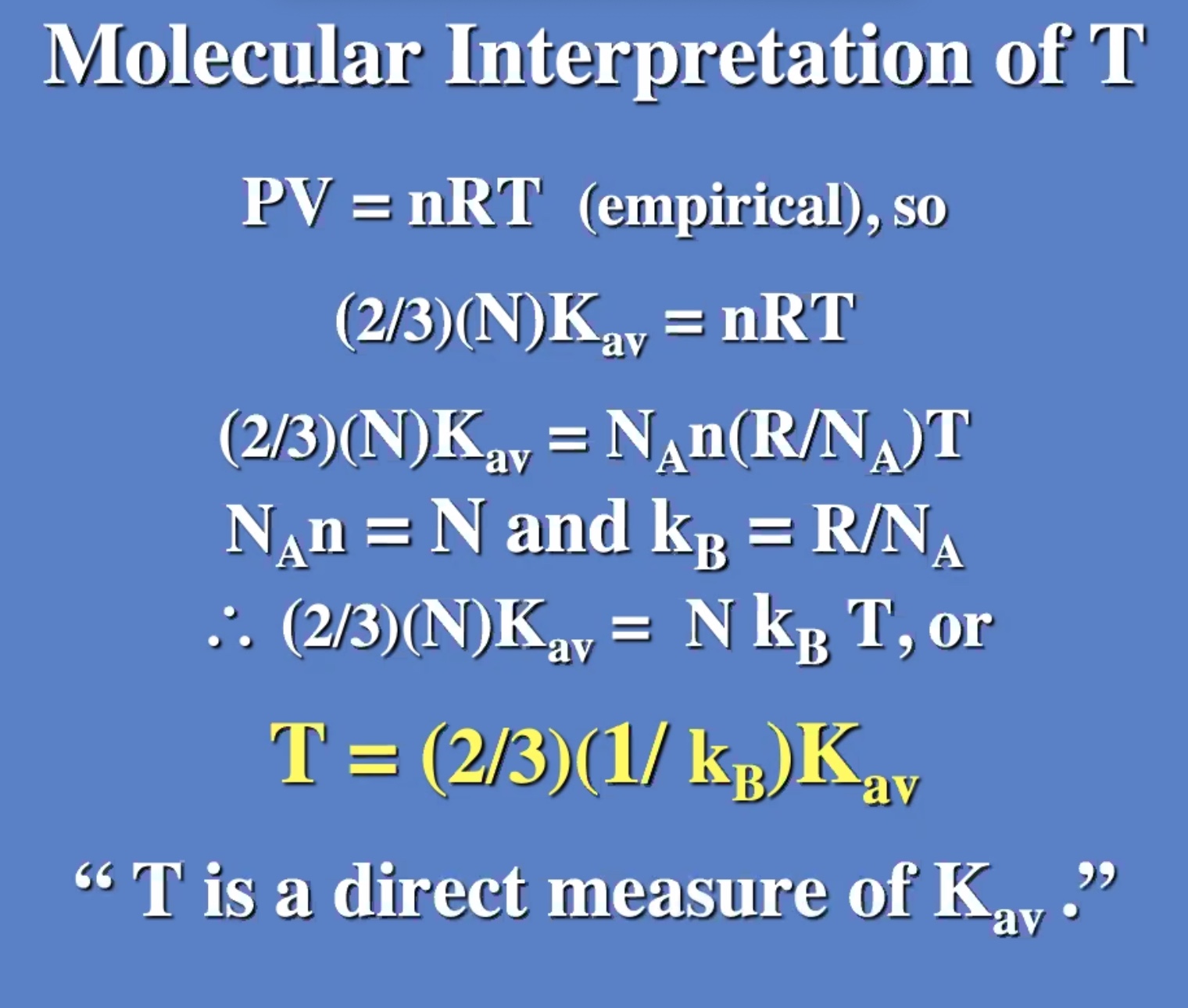
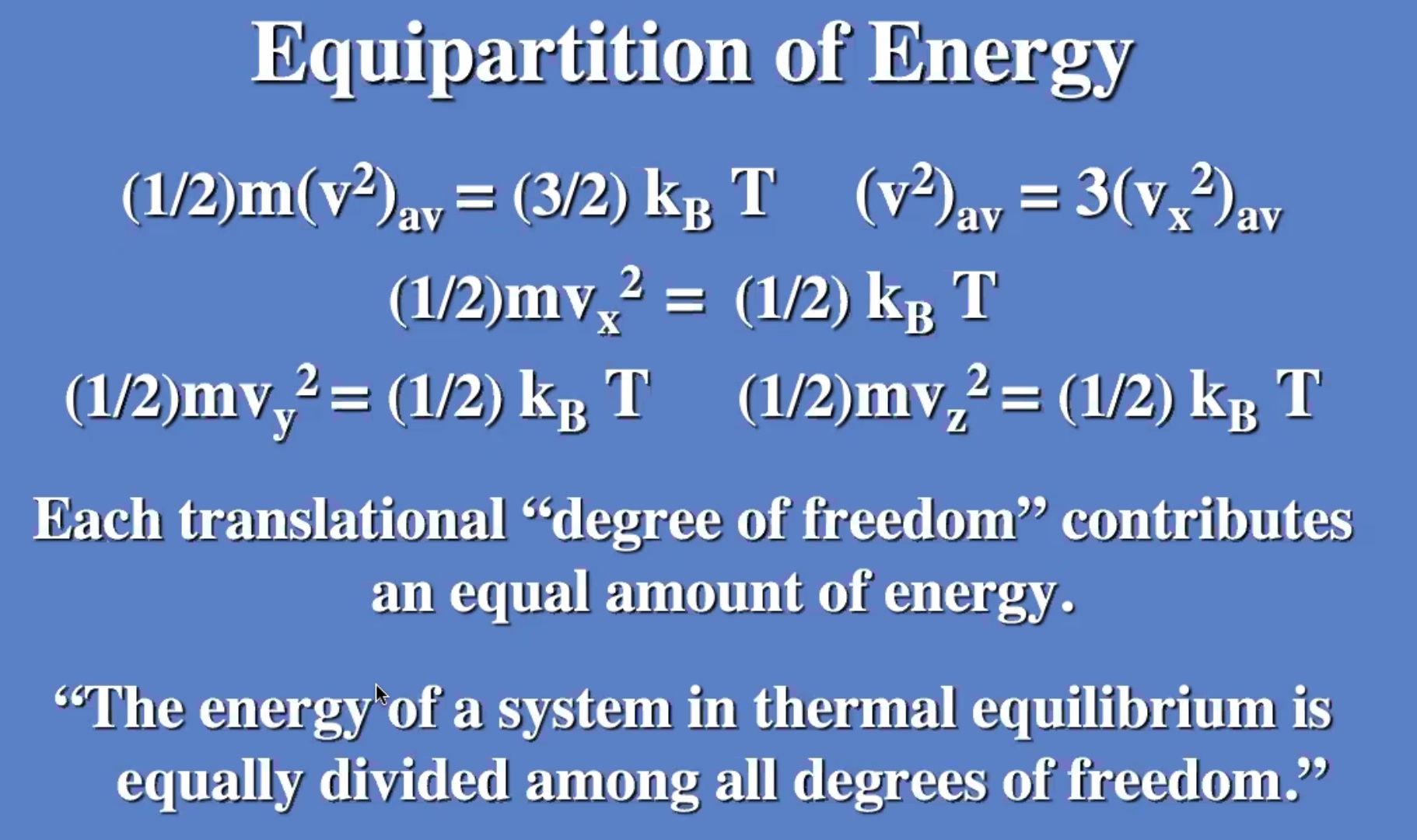

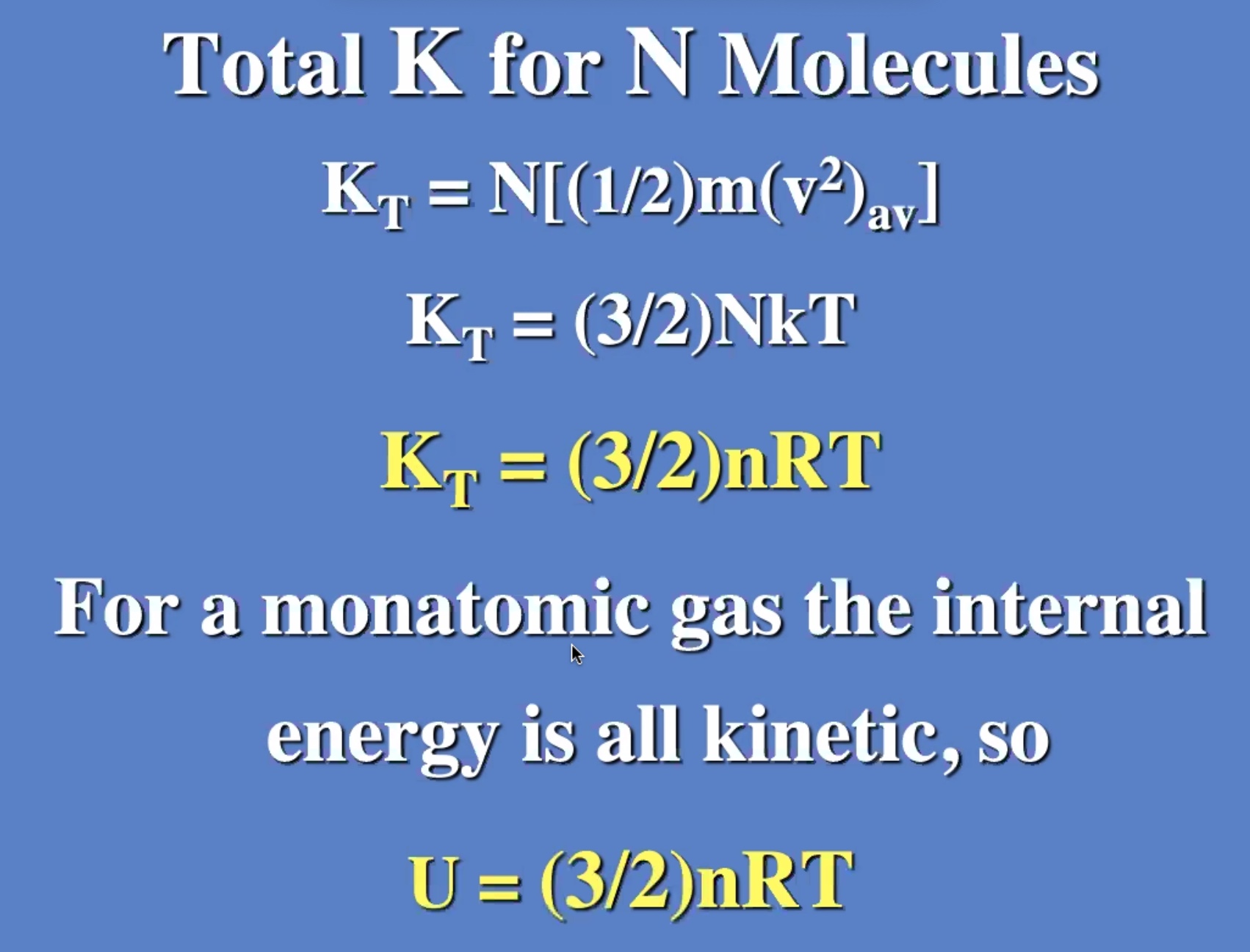
These don’t need to be derived, but understood. Will be useful on the next exam.

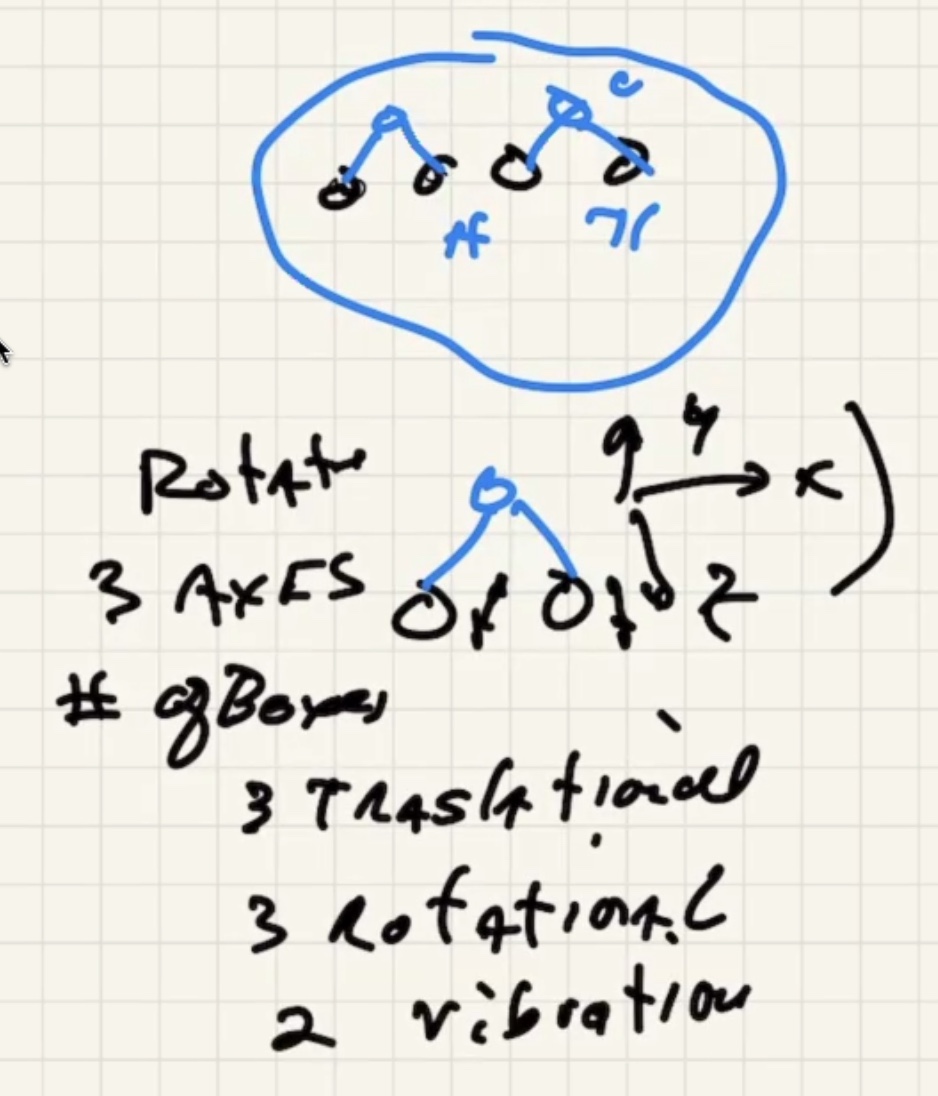
The degree of freedom is the “number of boxes” you can divide the energy into.
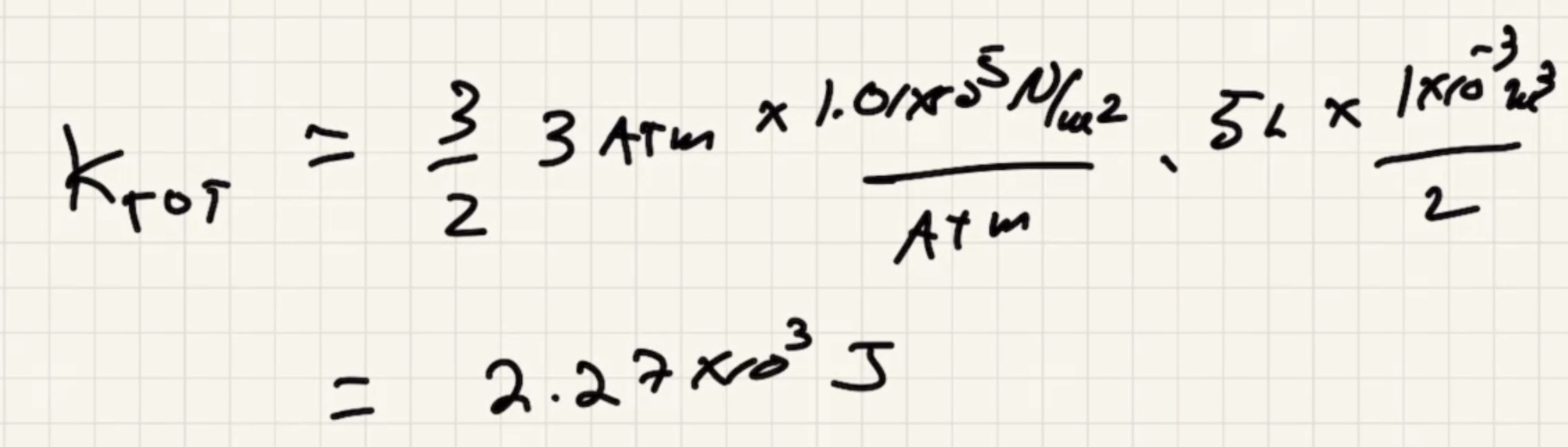
Example #

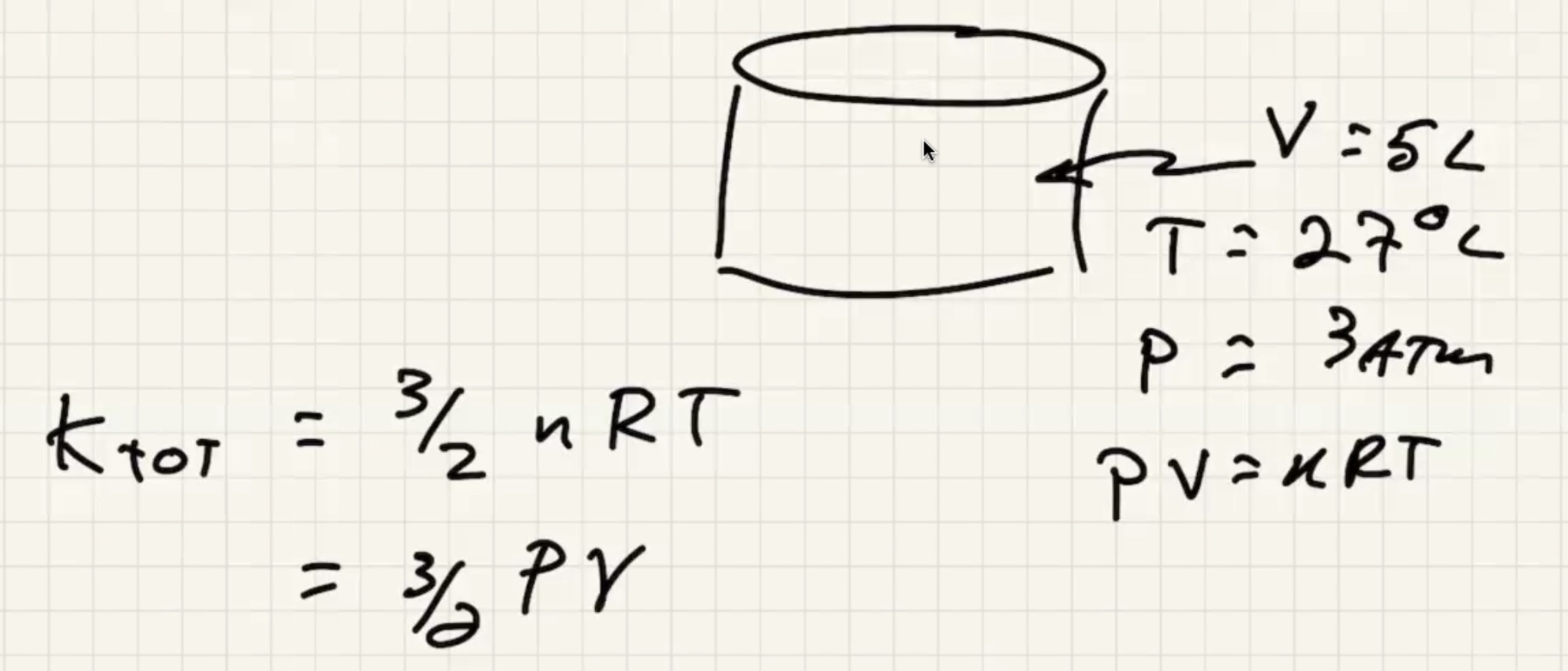
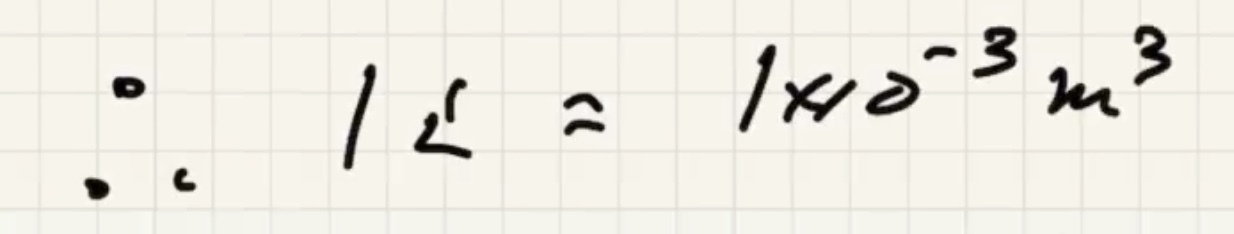
The conversion of a liter:
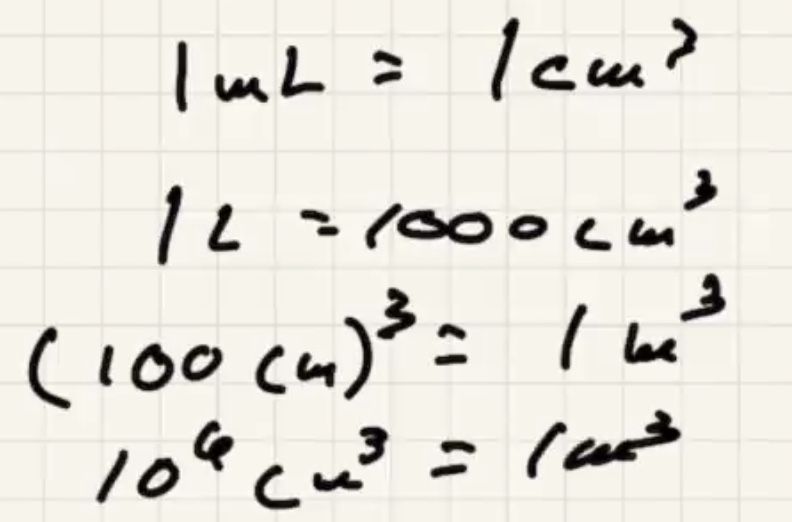
First law of thermodynamics #



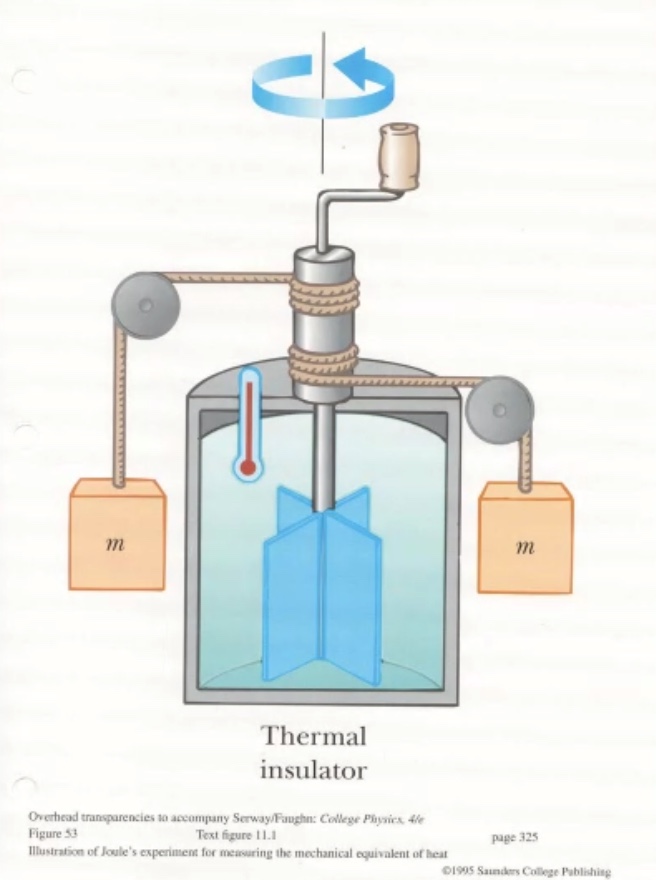

We’ll use the symbol (Q) for heat.

The change in internal energy equals the heat added to or the work done on the system.


Imagine dumping a bunch of heated copper pellets into a beaker of water and stir:
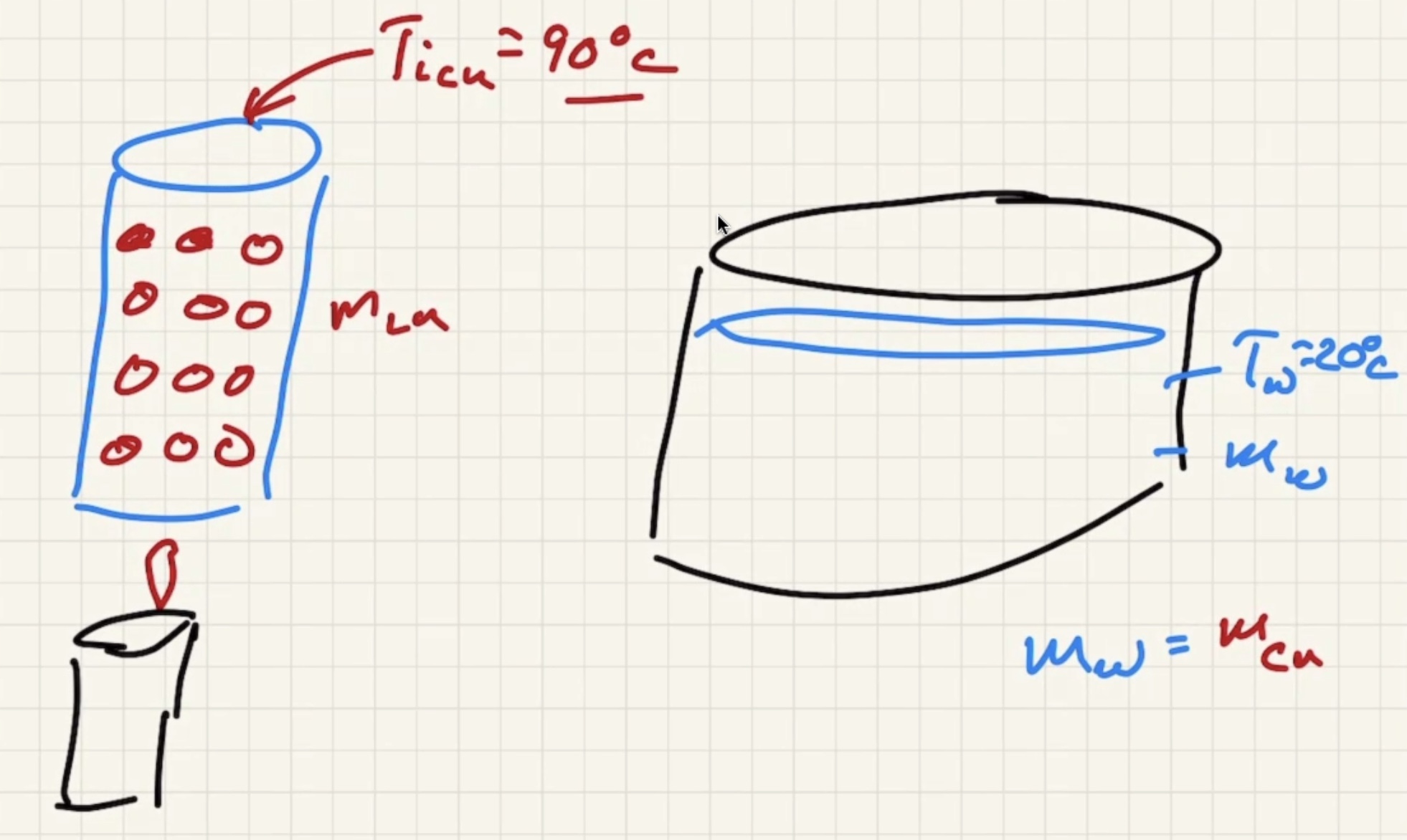
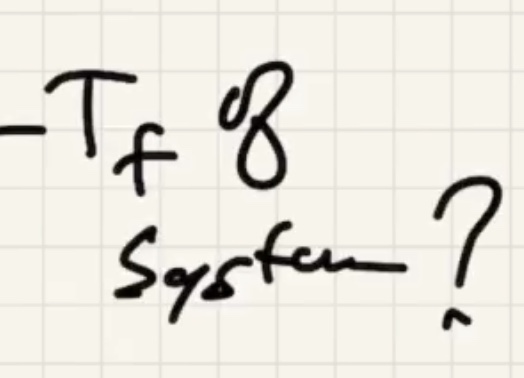

The final temp ends up being close to the water temp:

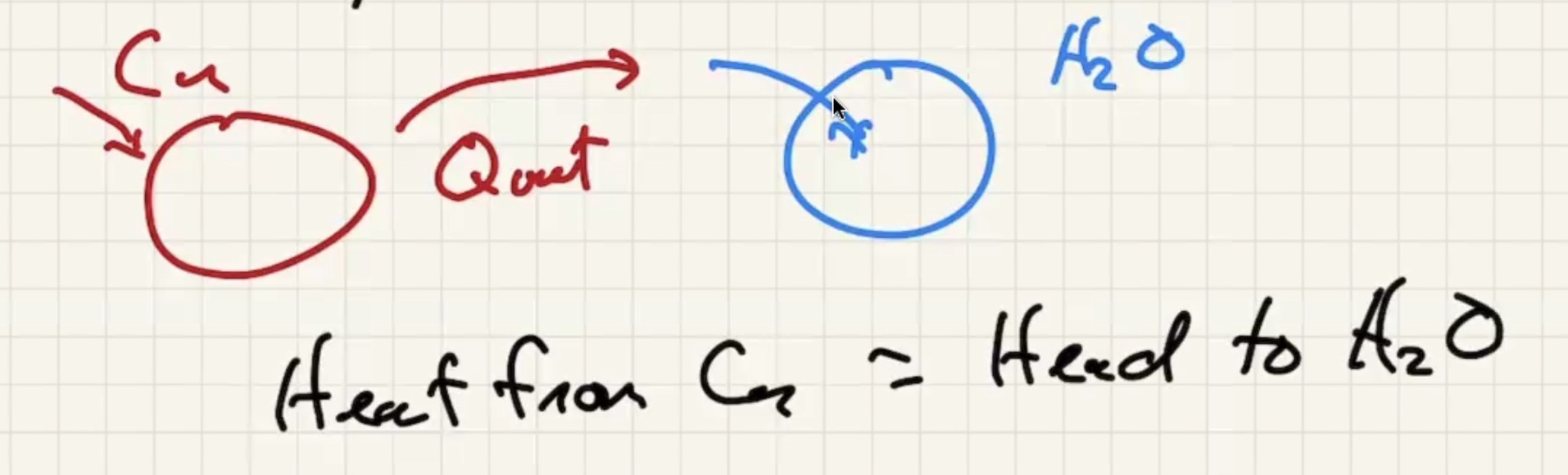

Remember water has an ability to absorb a lot of heat without changing temp.
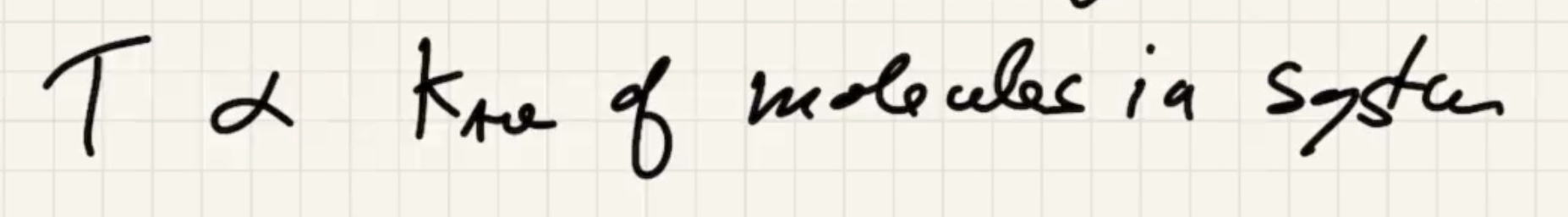
Looking at the copper against a thermometer:
Looking at water against the thermometer:
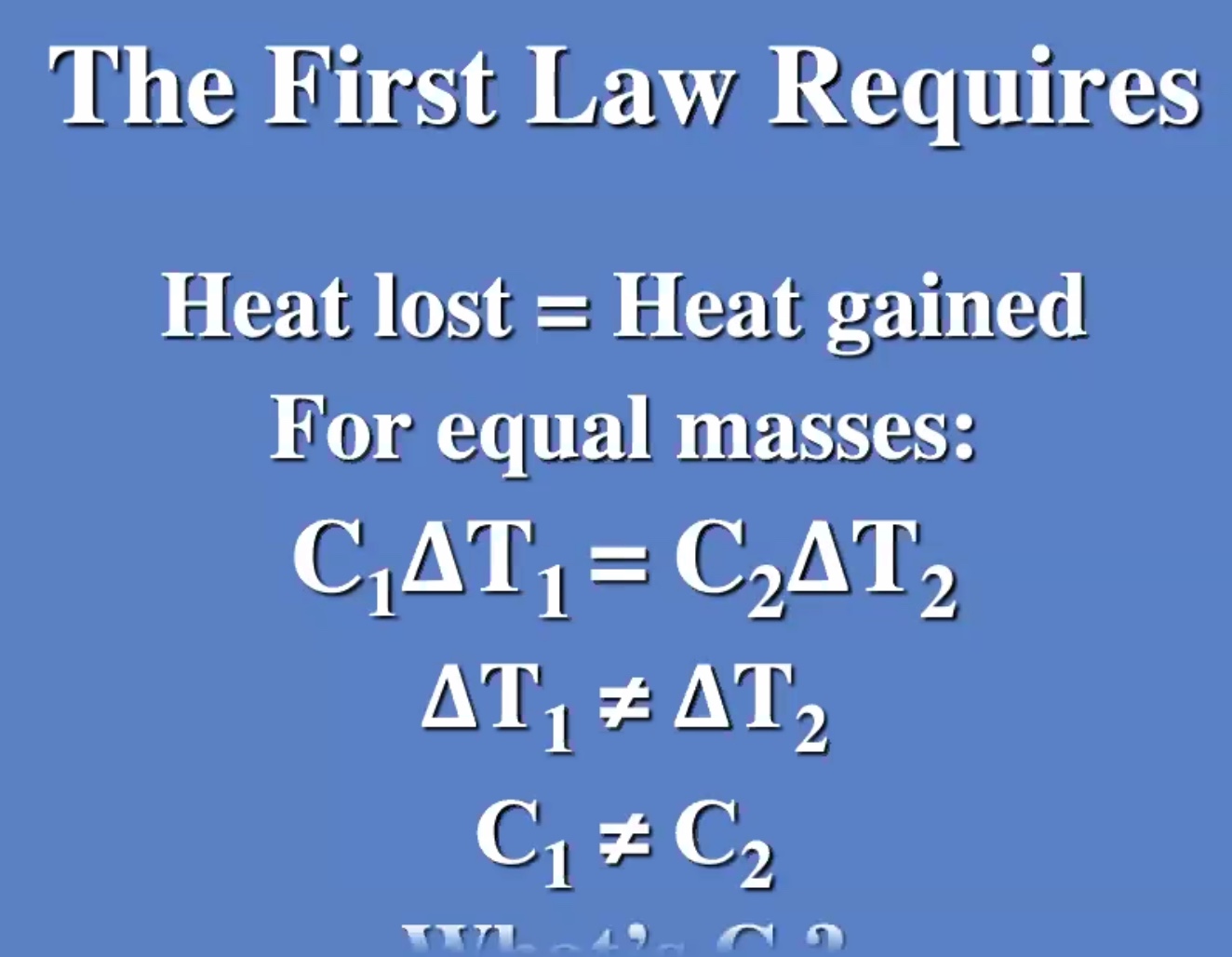
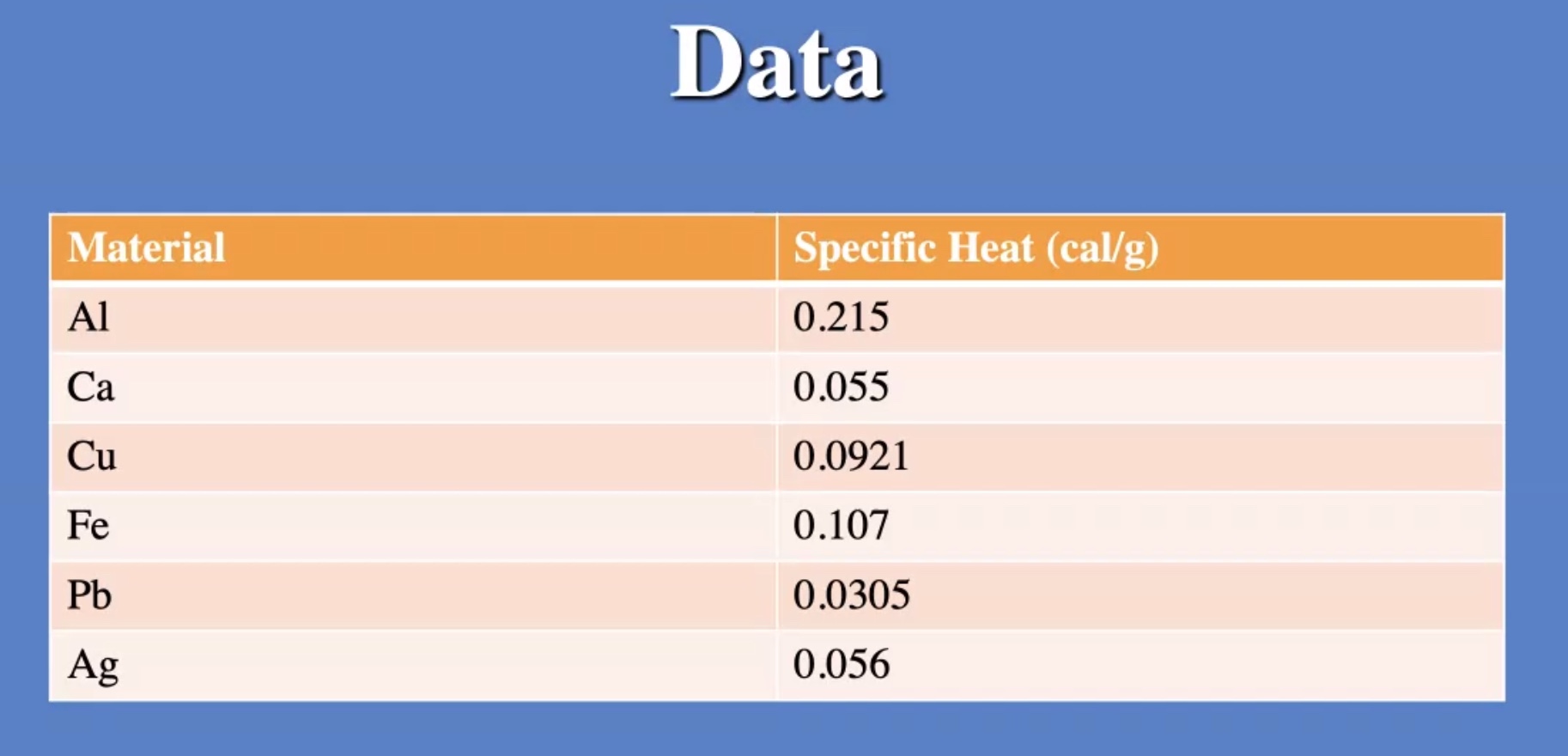
Whats (C)?



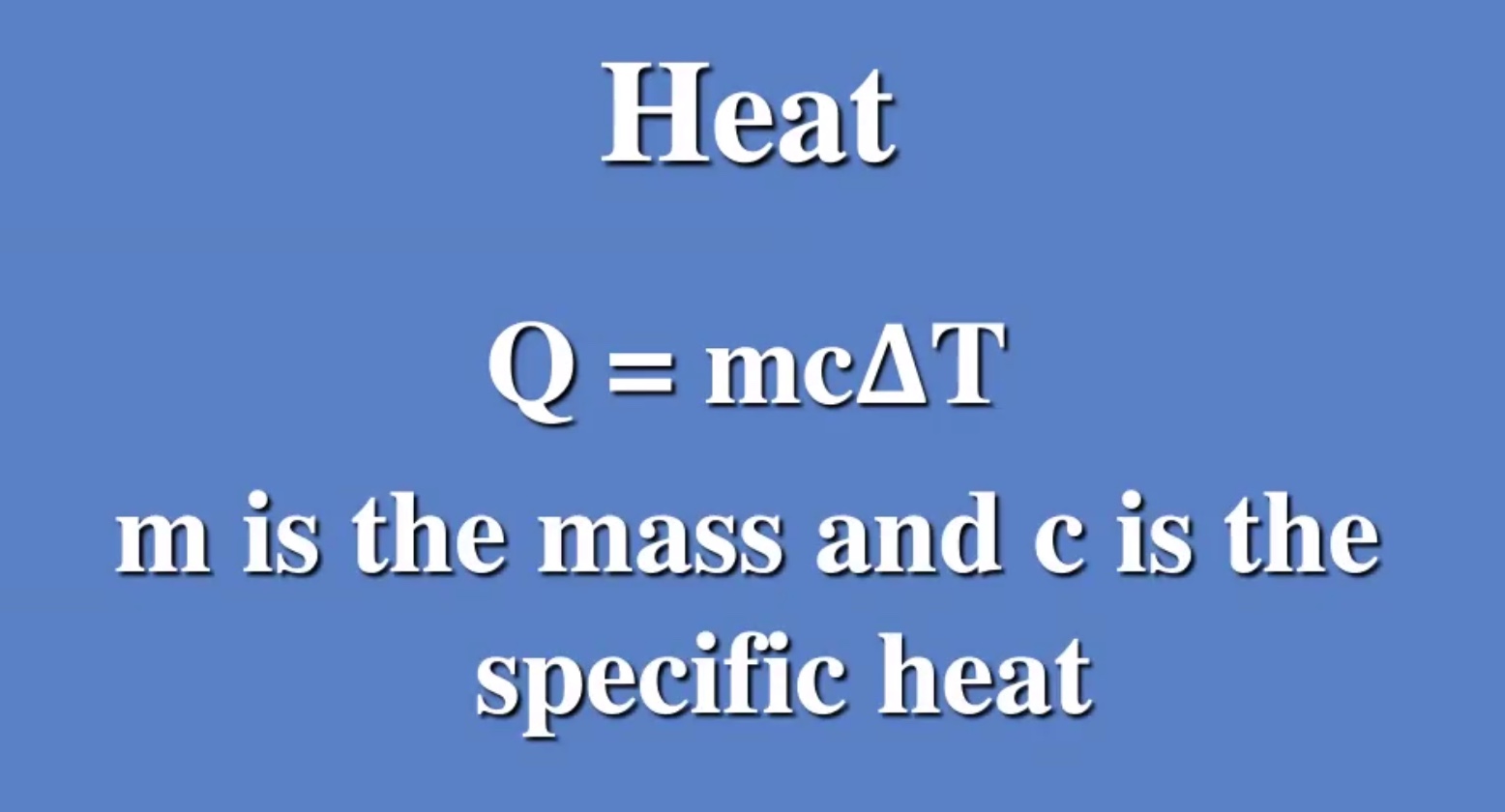
Example #

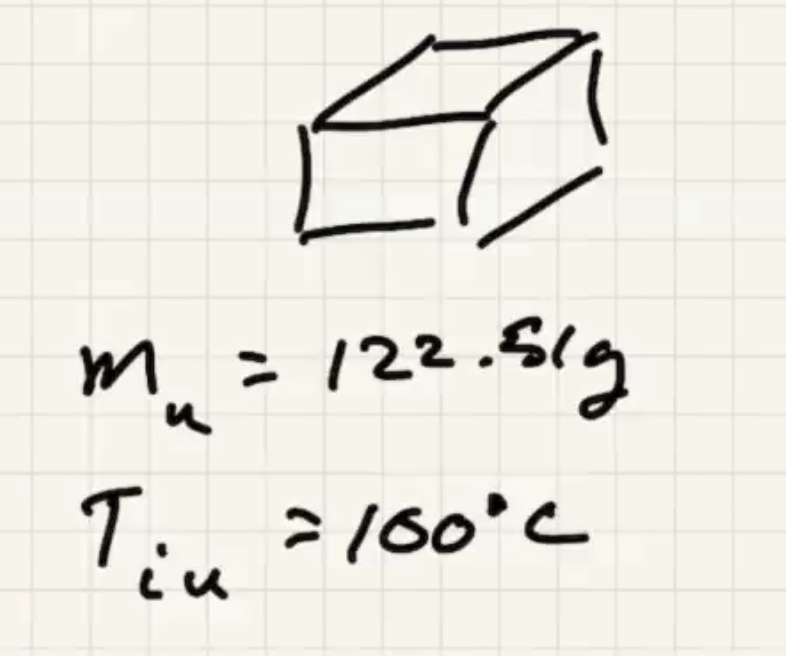
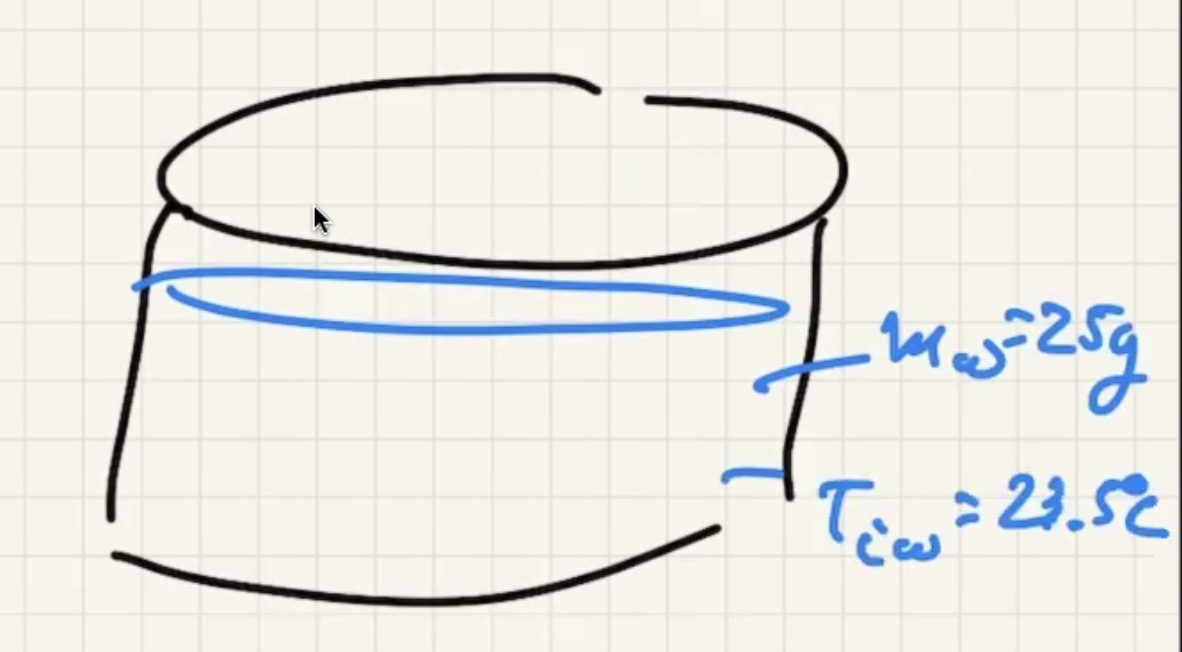
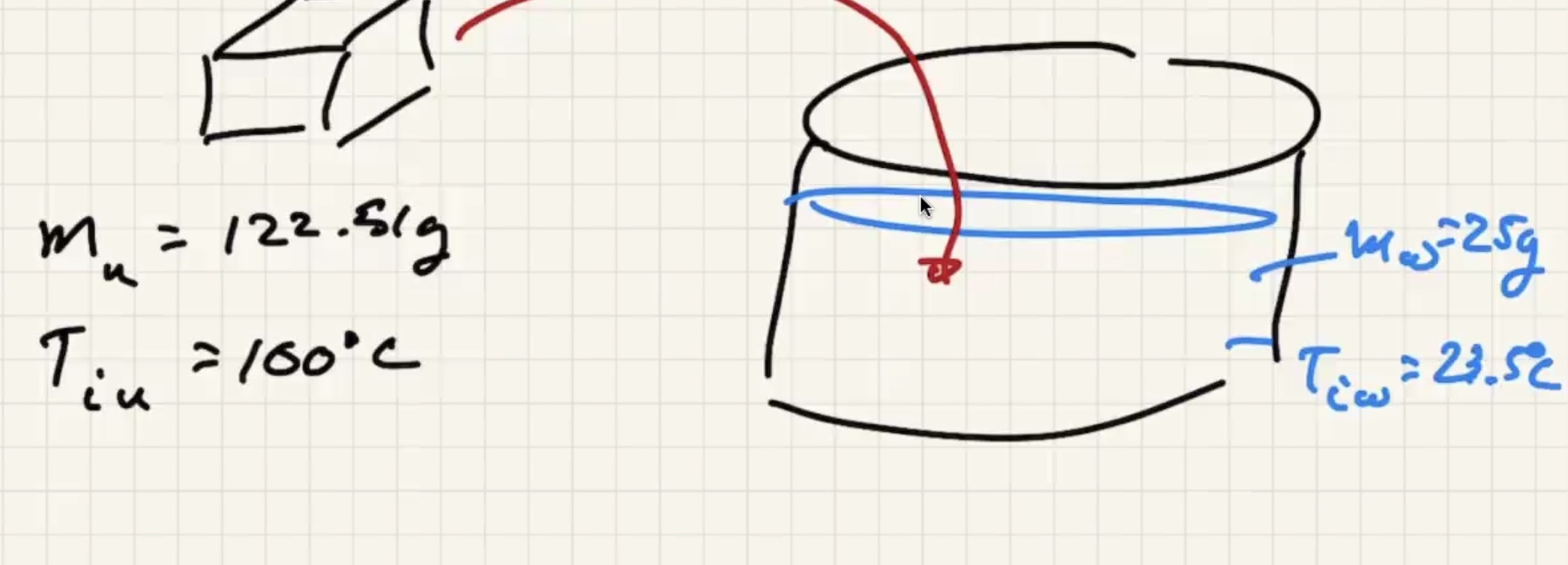
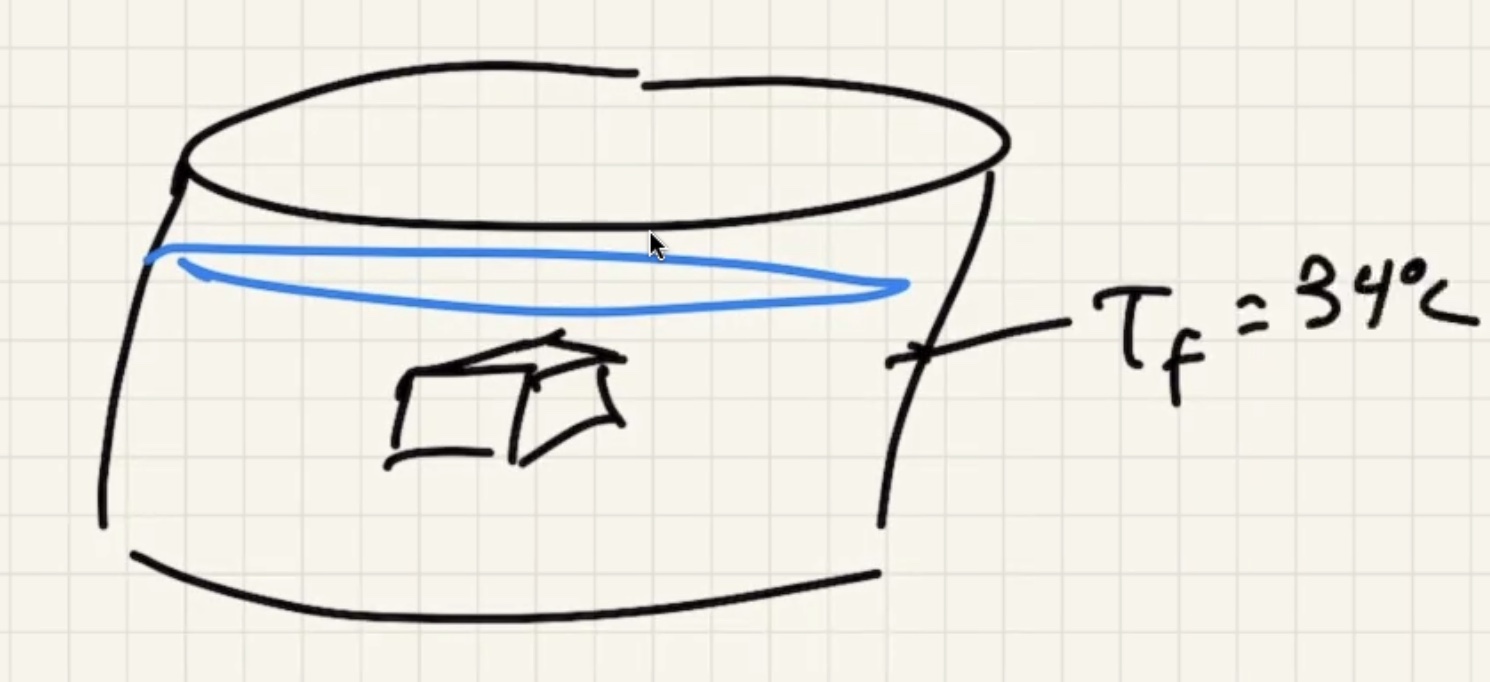
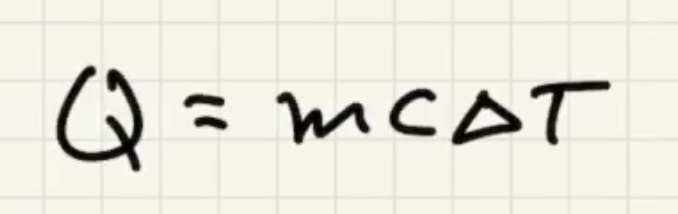

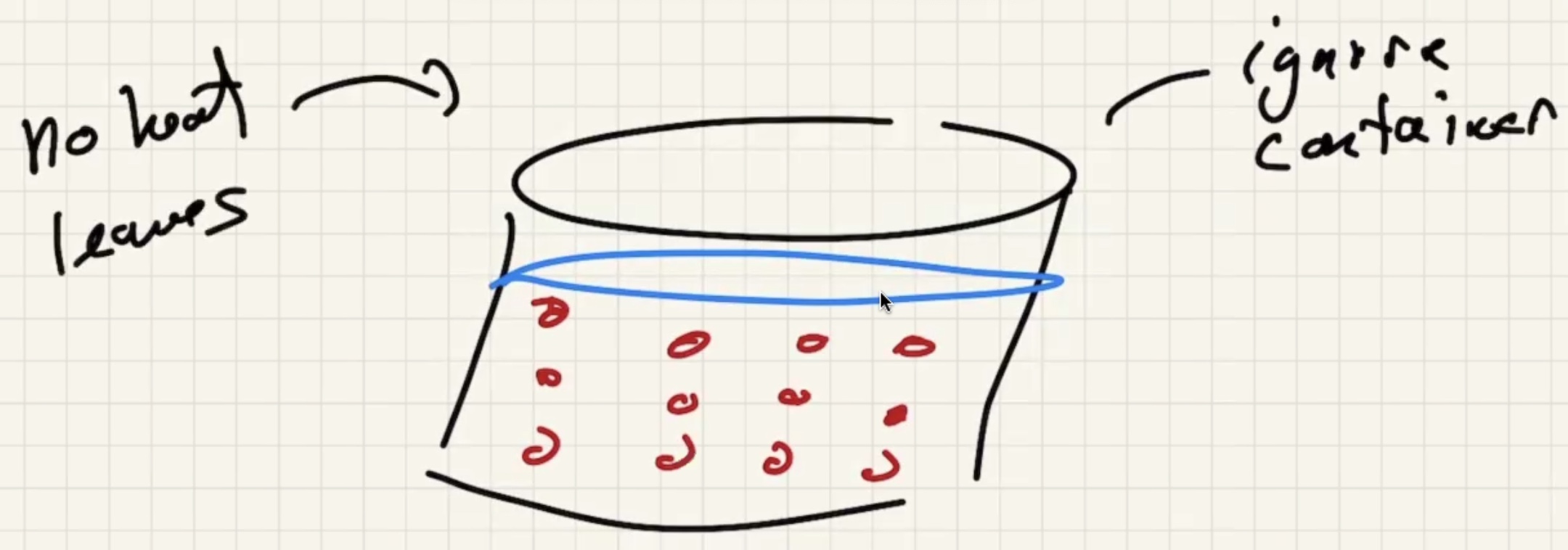
Ignore heat exchange with the calorimeter/outside world.


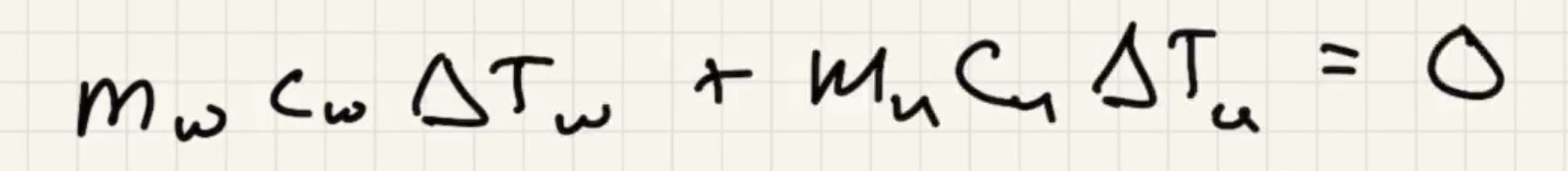
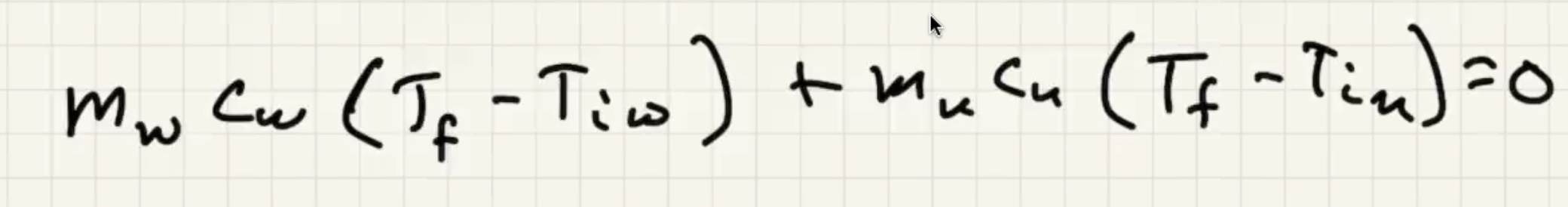
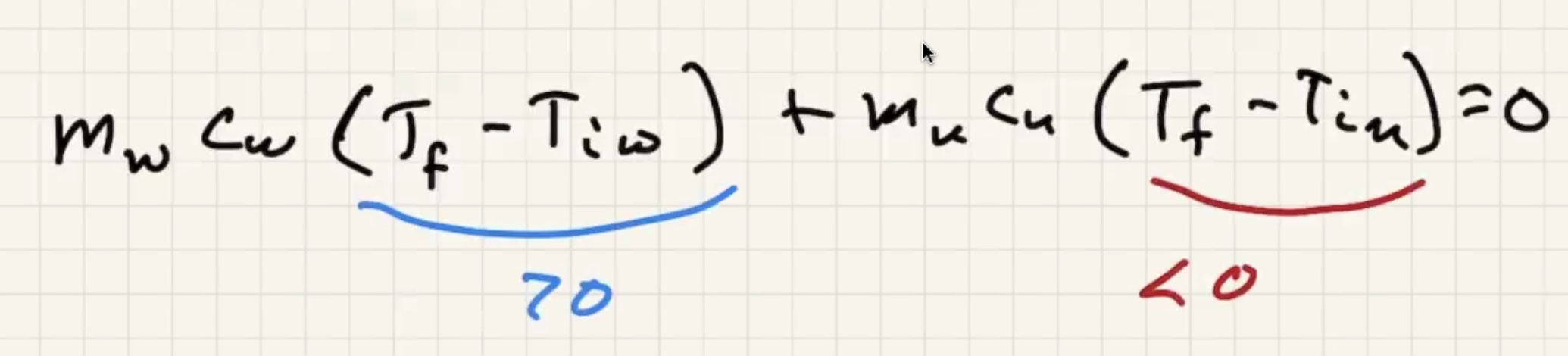
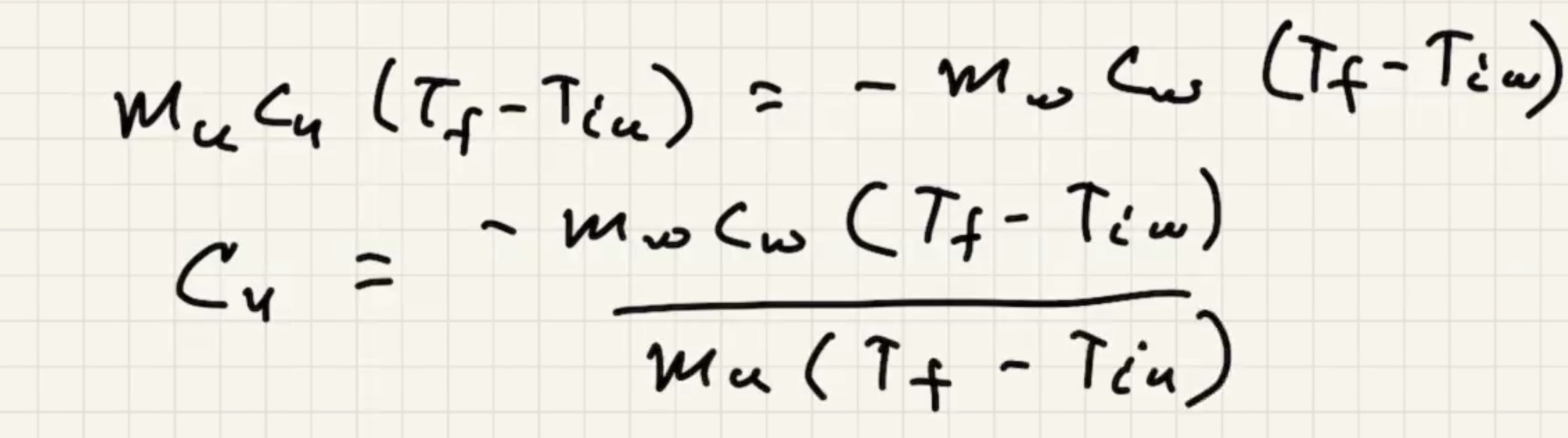
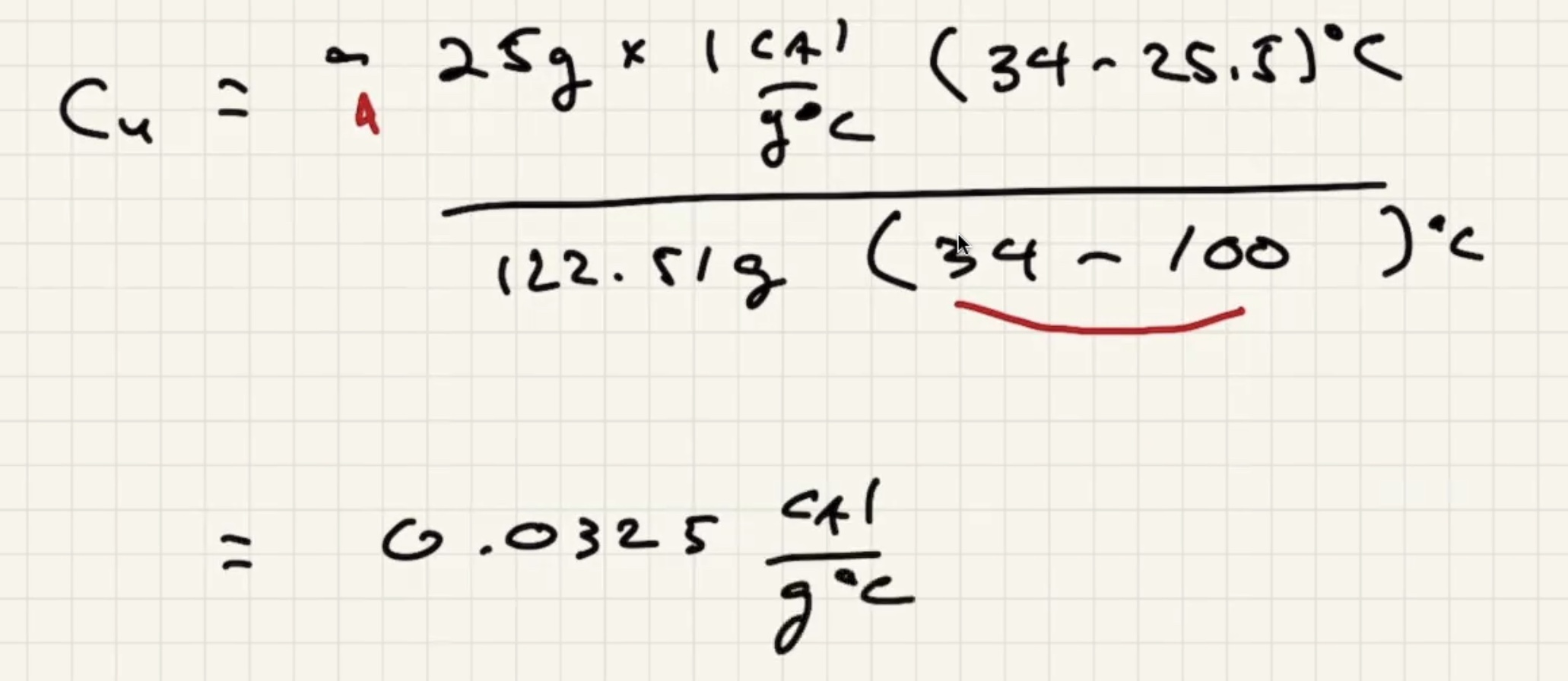
The answer we got for our grey metal is very close to lead: