Announcements #
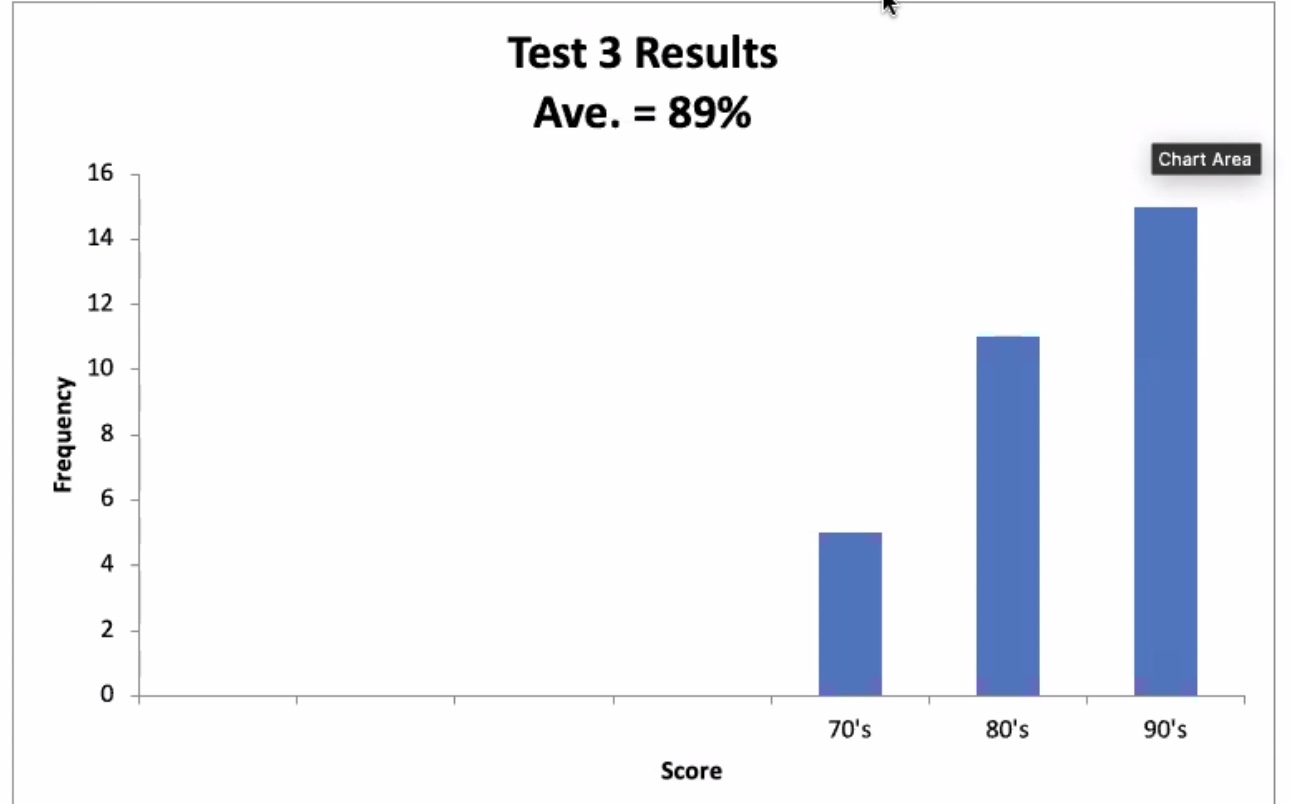
Test scores

Probably cancel today’s recitation.
Today #
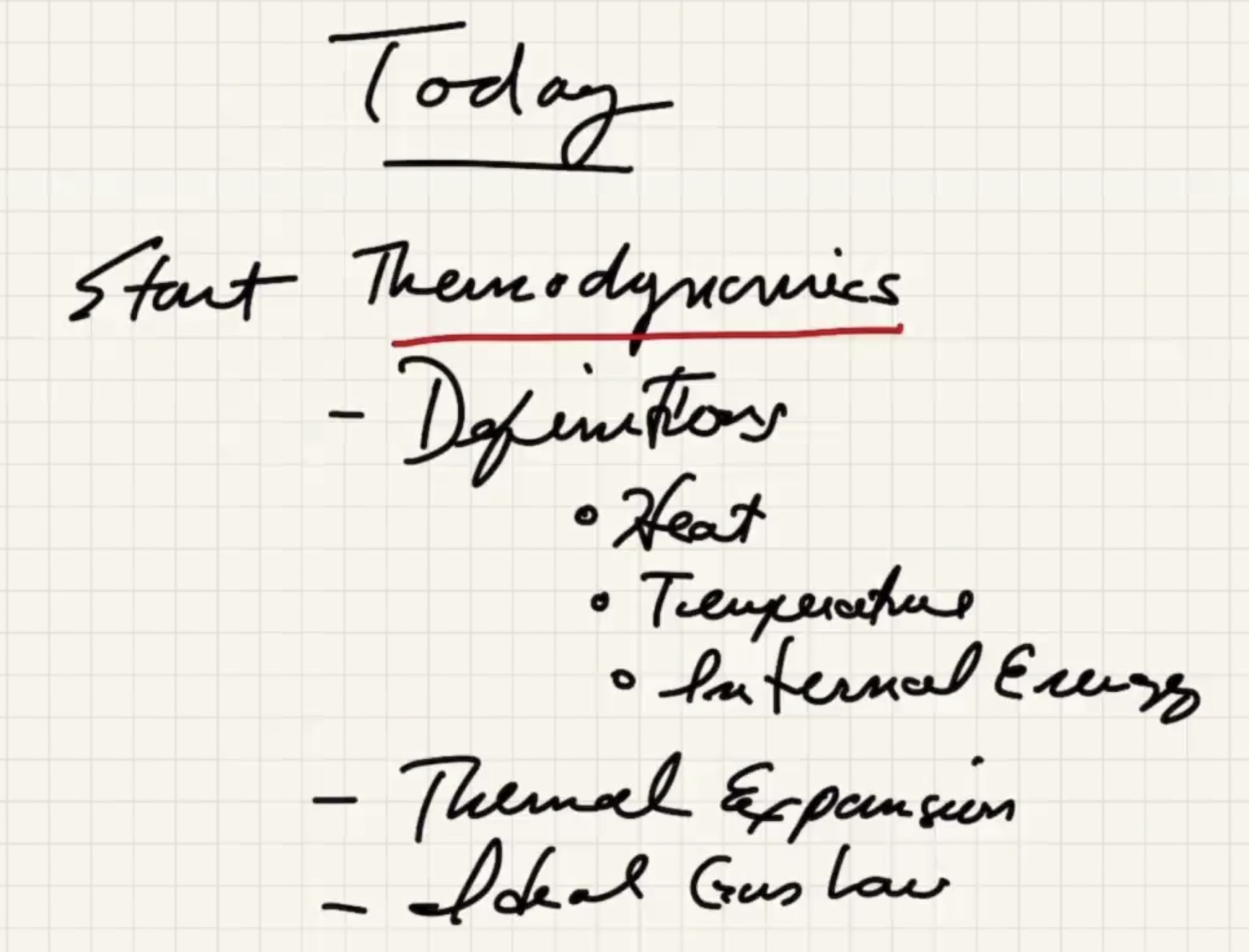
Microscopid mechanics will have macroscopic ramifications. We’ll have to go over:
- Heat
- Temperature
- Internal energy
- Thermal energy
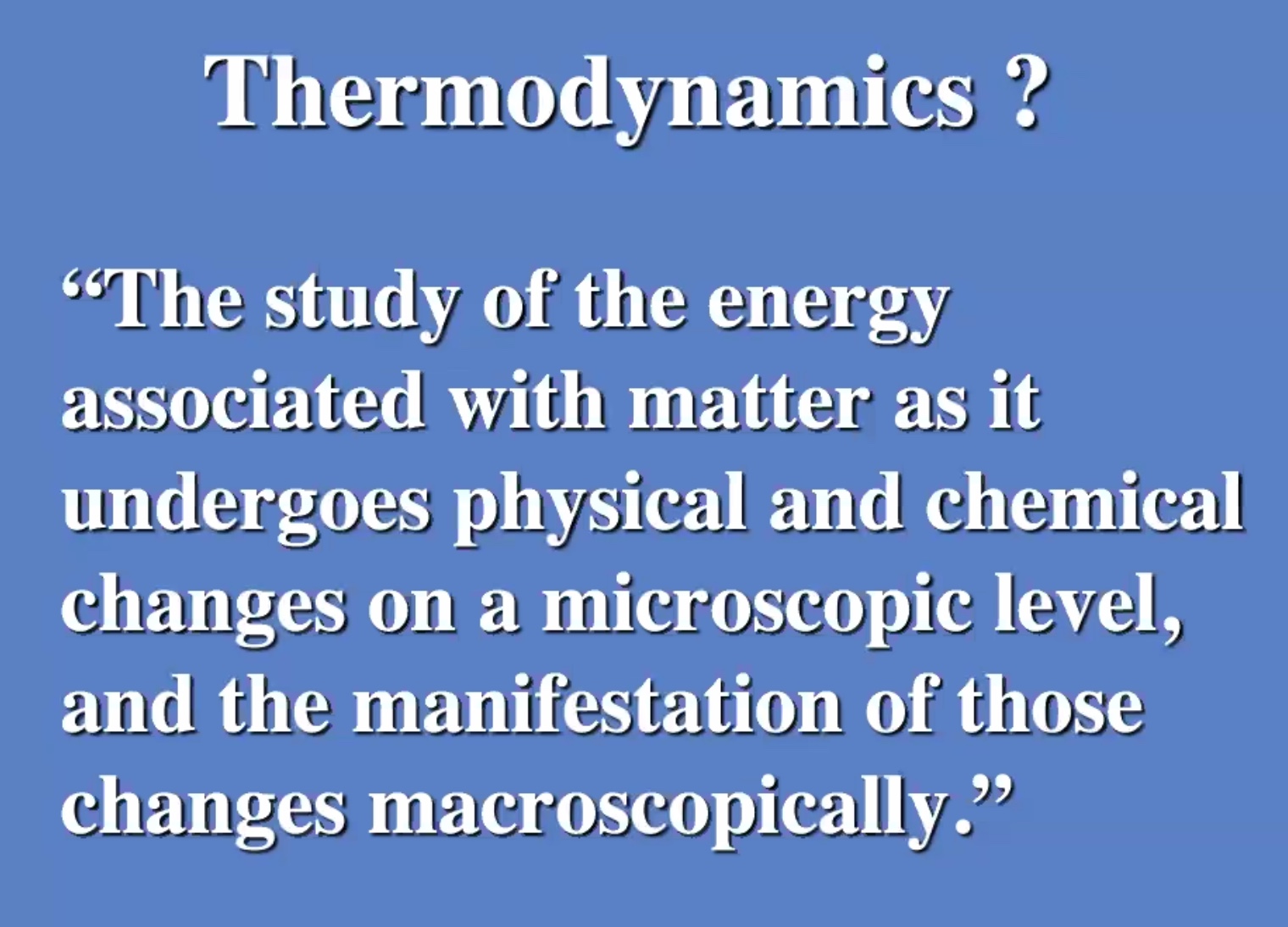


Temperature #
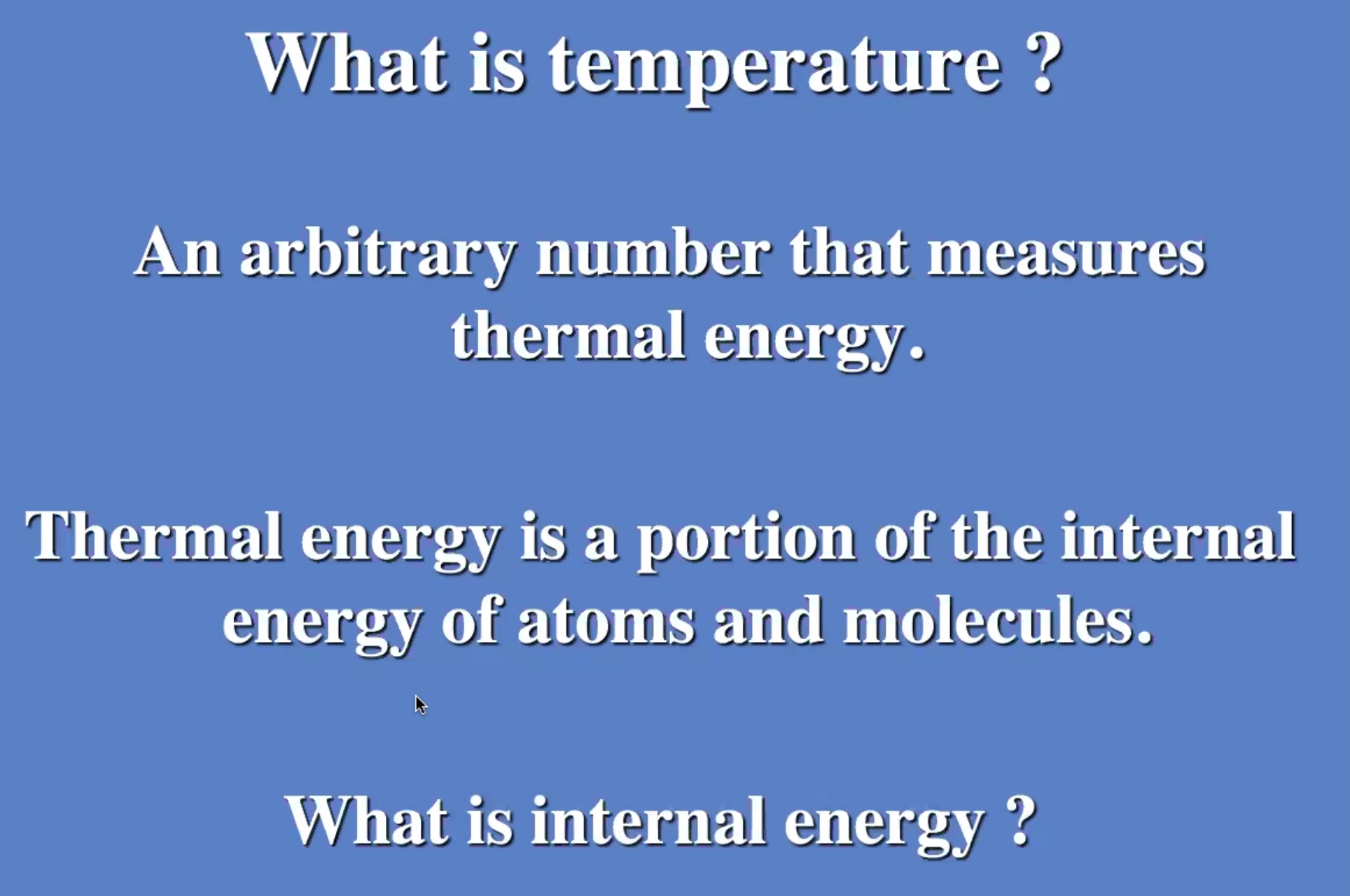

Prof Harris will almost always use (U) for internal energy instead of (E).
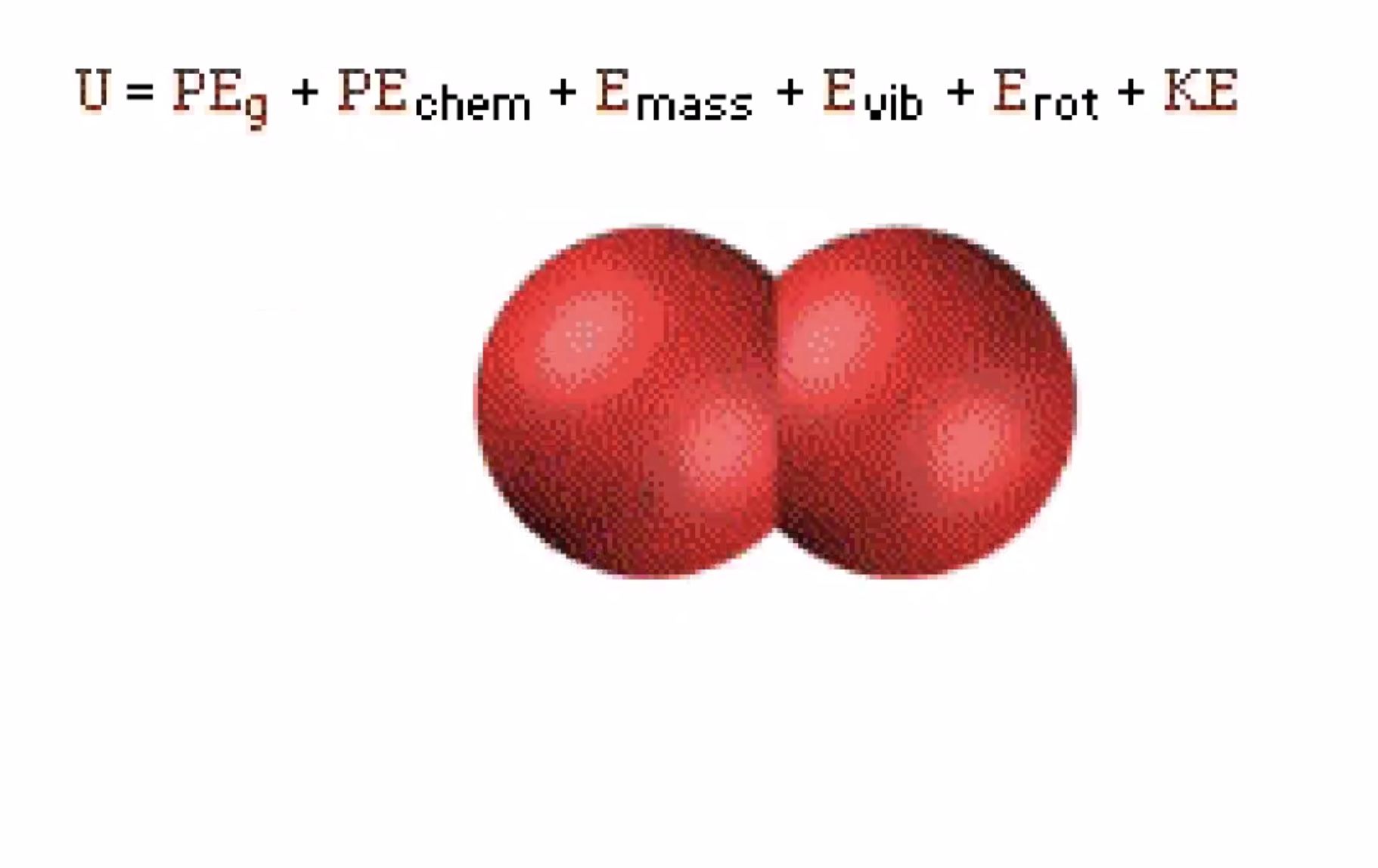
Temperature affects the vibrational energy (E_{vib}), rotational energy (E_{rot}), and the kinetic energy (KE).
Thermal energy
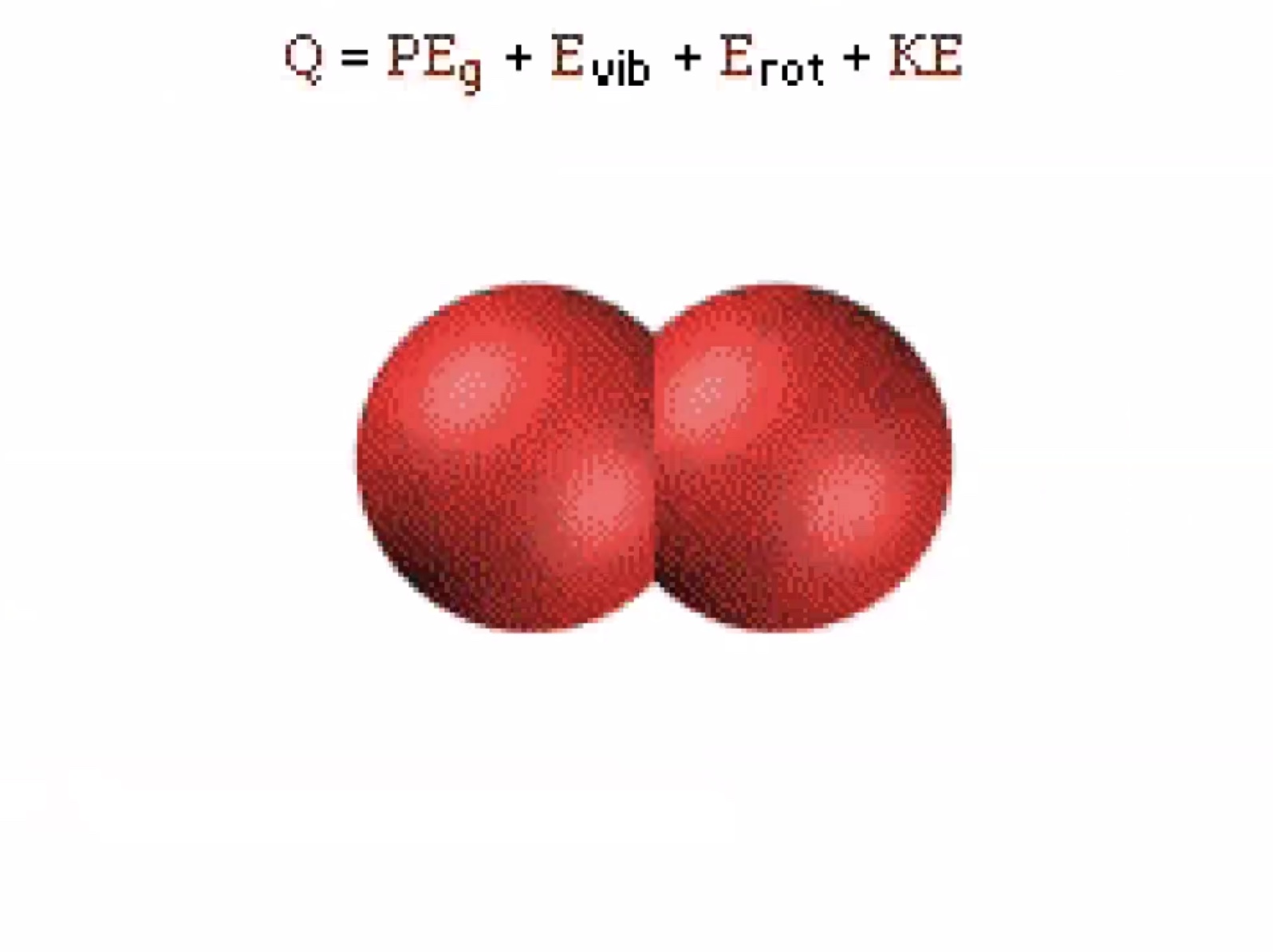
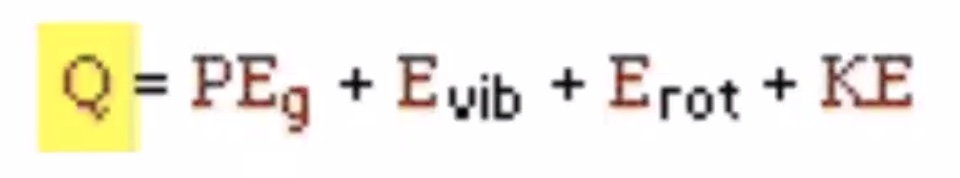
The aspects of the internal energy that is affected by thermal processes. The thermal energy (Q) at room temperature the translational kinetic energy is usually much greater than all the other forms of energy.

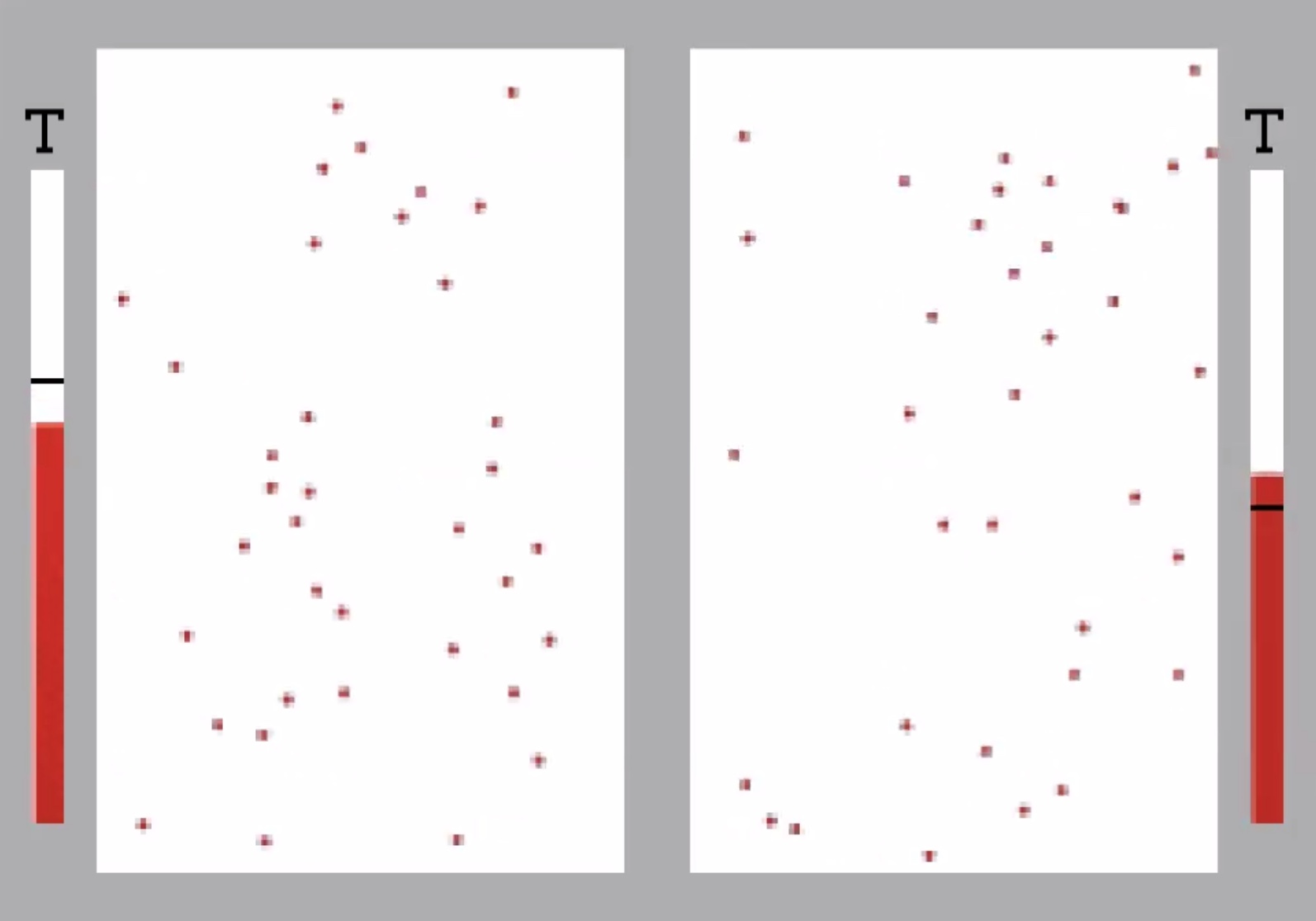
Thermal energy can be transmitted thru the walls of the container by vibrating the molecules of the wall. The heat can be transferred between each gas thru the wall.

Temperature is the measure of the average molecular energy of the substance.
Thermal equilibrium is when the average temperatures between two gases are the same. It doesn’t matter how many molecules of gas there is.

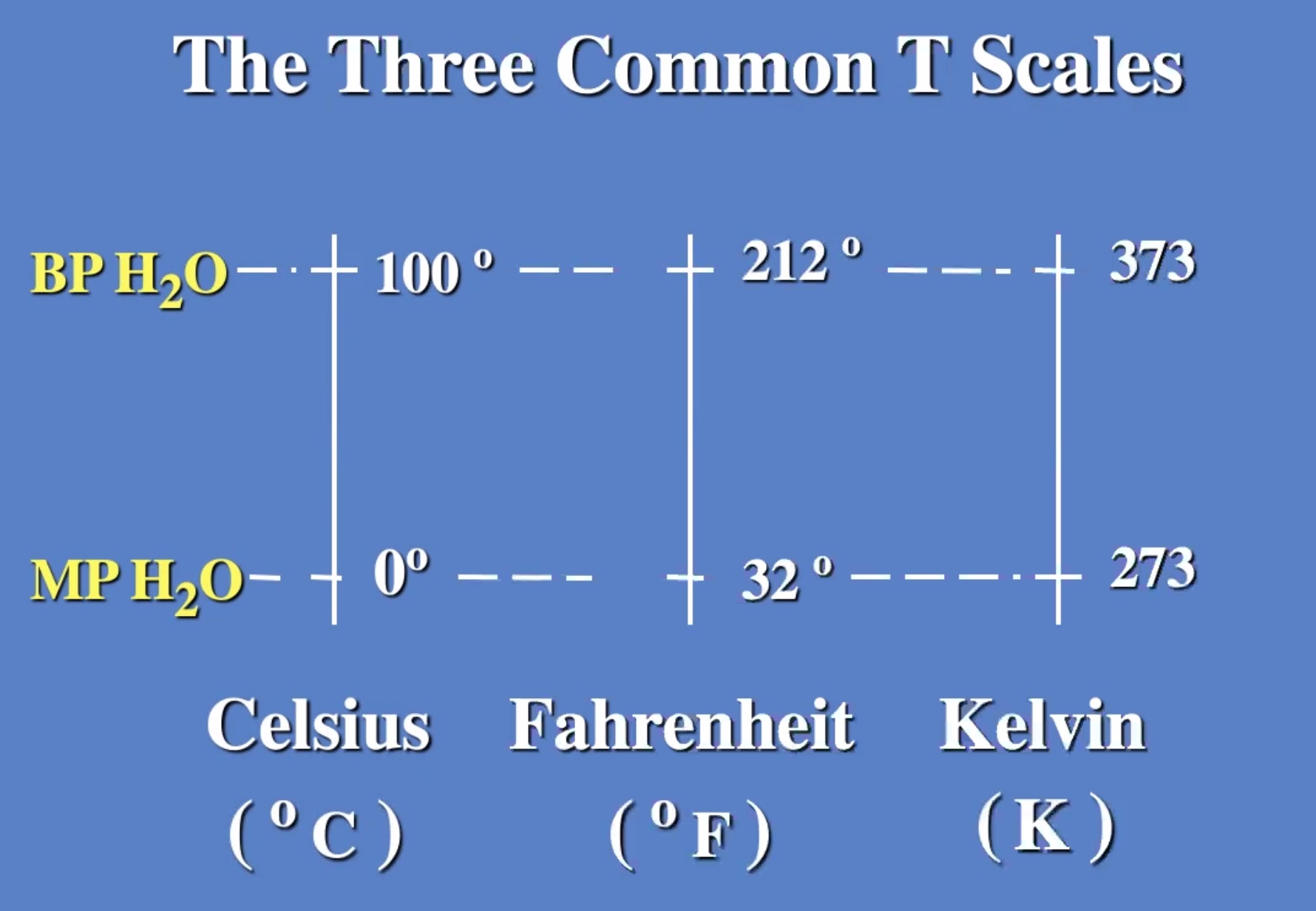

Thermal equilbrium is like the transitive property in algebra.
If (A)
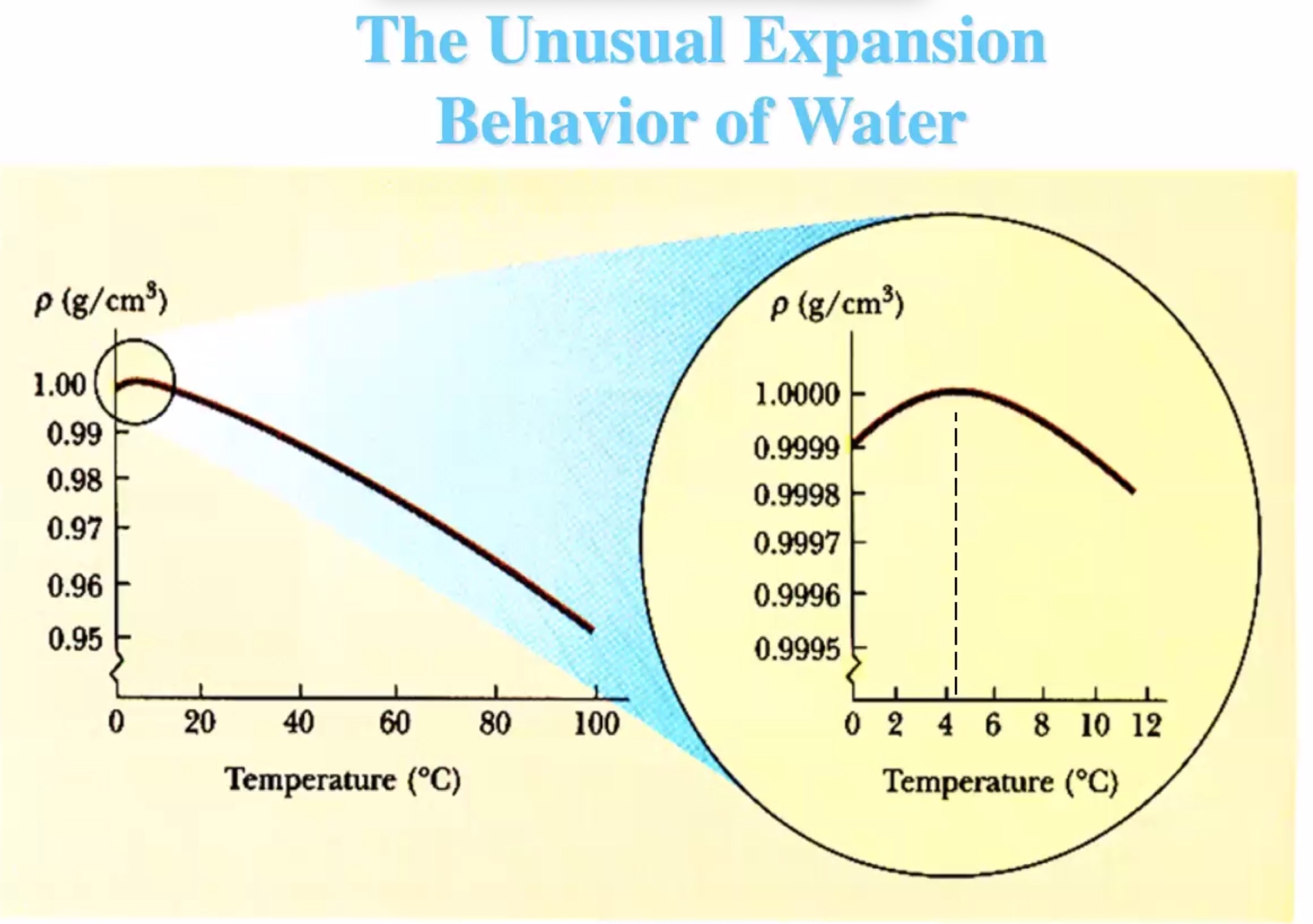
Thermal expansion #

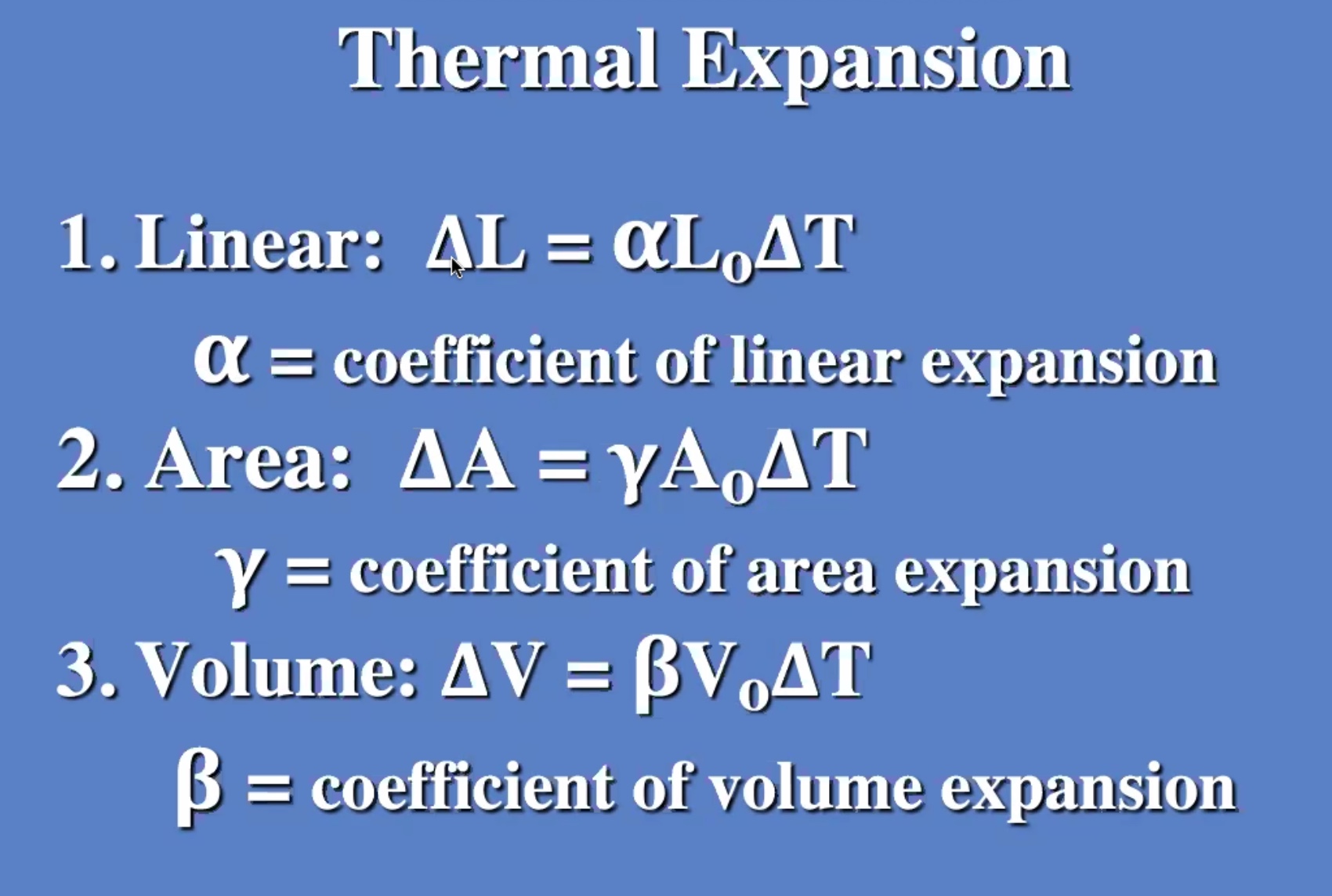
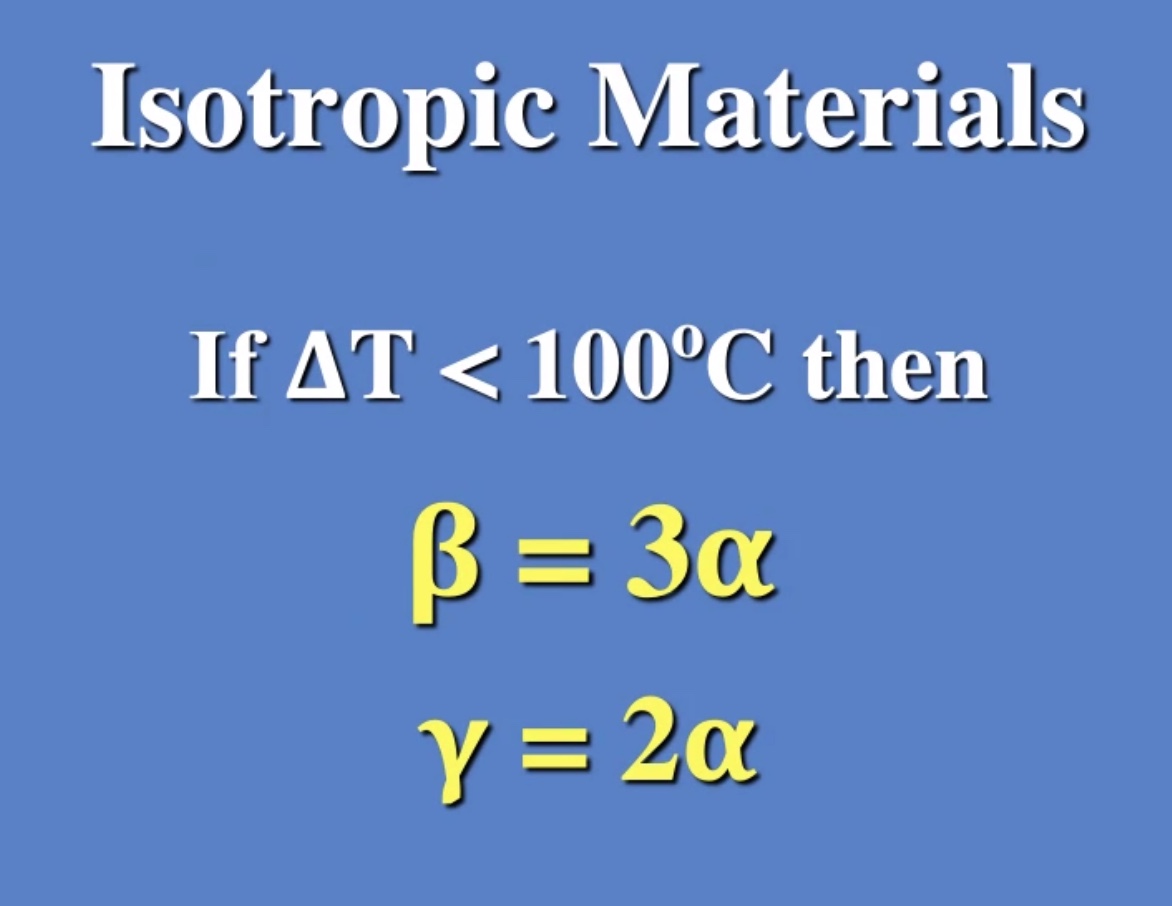
Here (\Delta T) is the change in temperature, (\alpha) is the coeffecient of expansion, (\gamma) is the coeffecient of area expansion, (\beta) is the coeffecient of volume expansion.

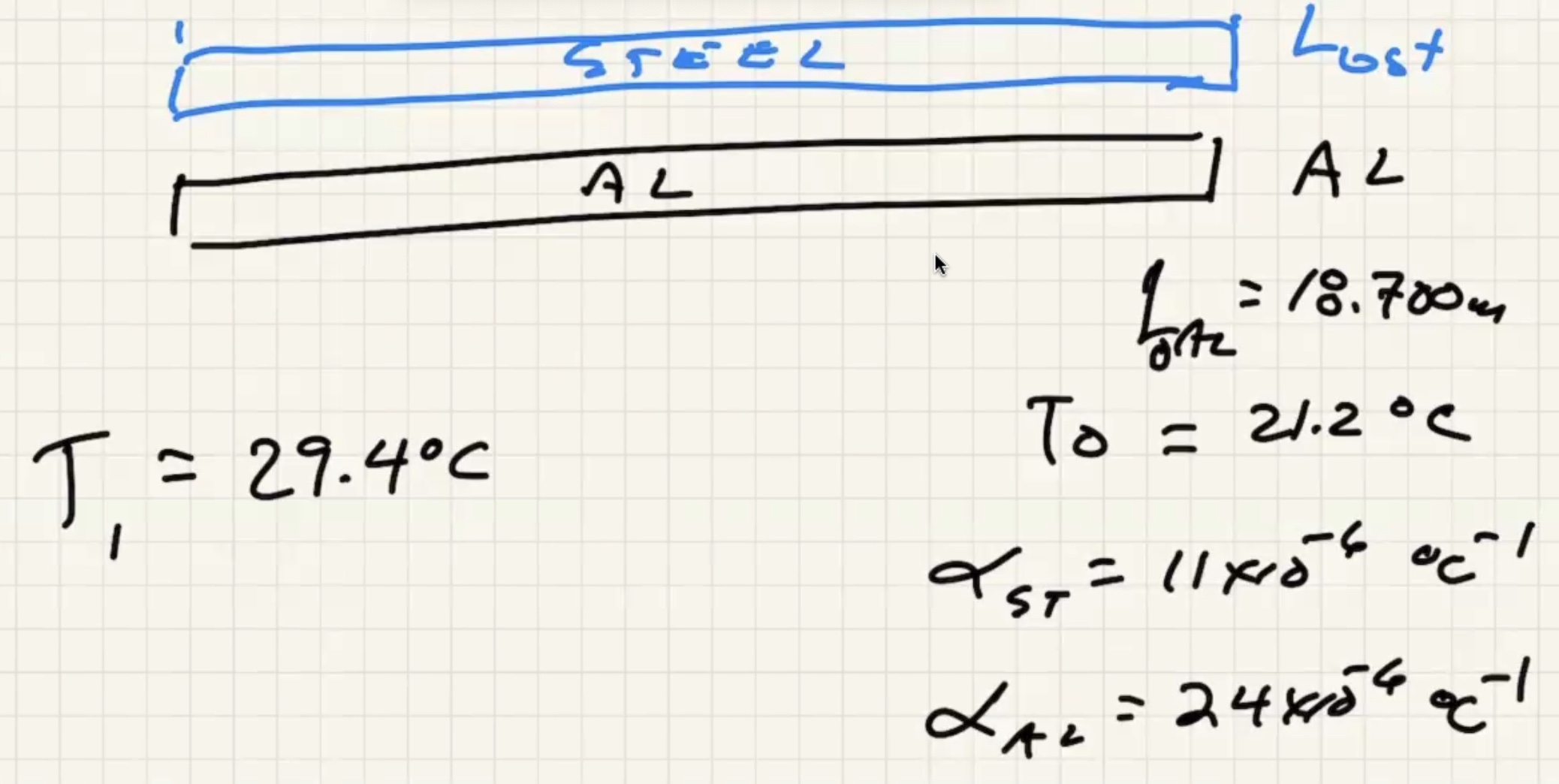
a)
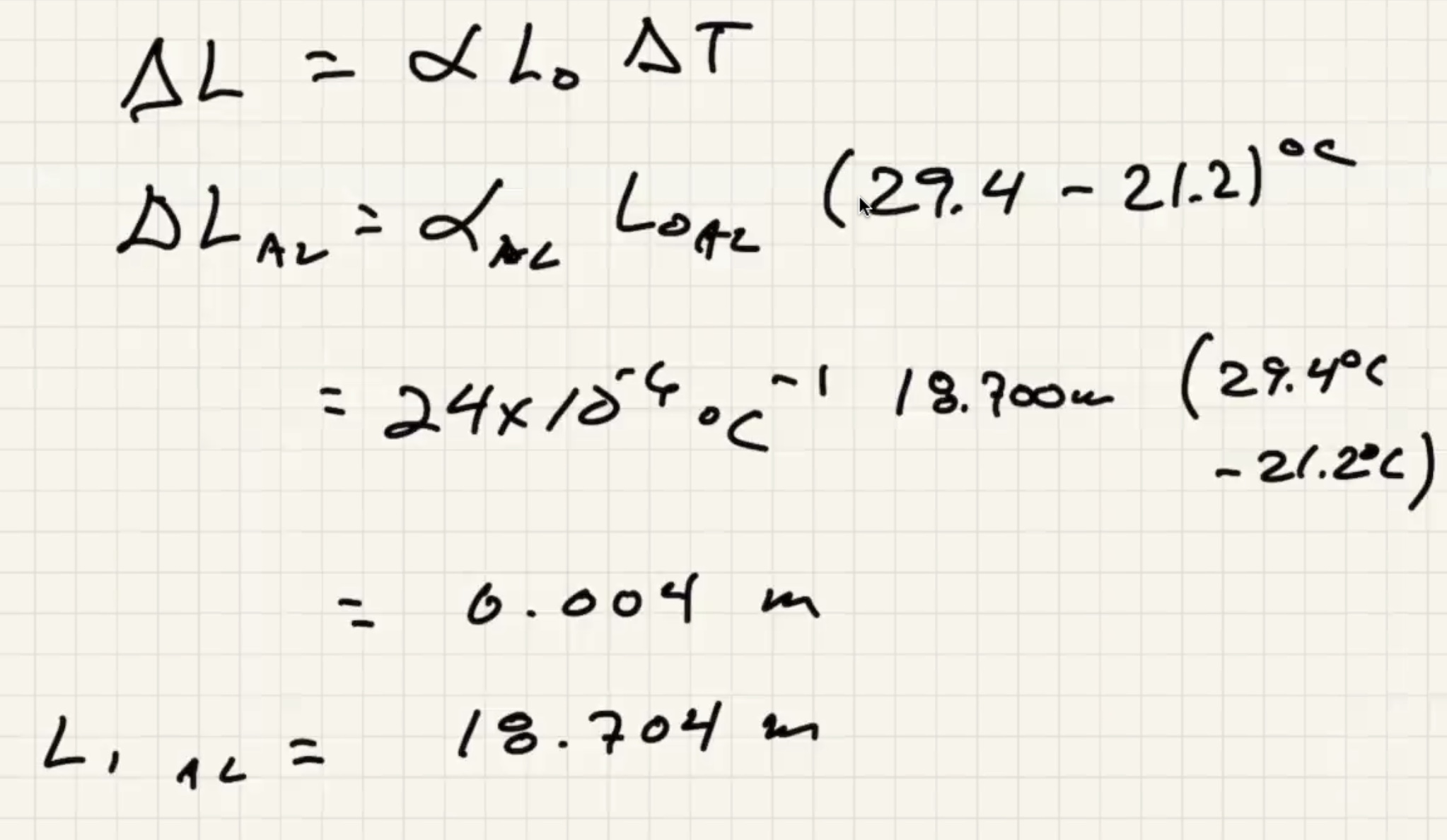
b)
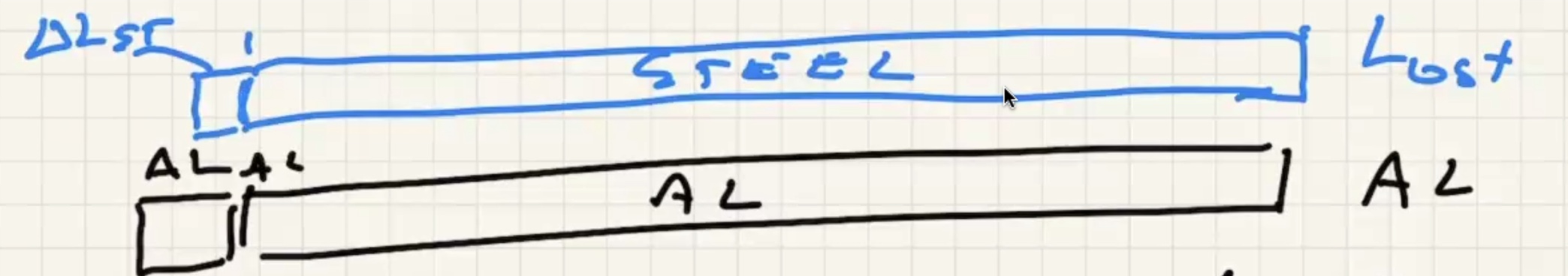
The measuring tape also expands with that change in temp.

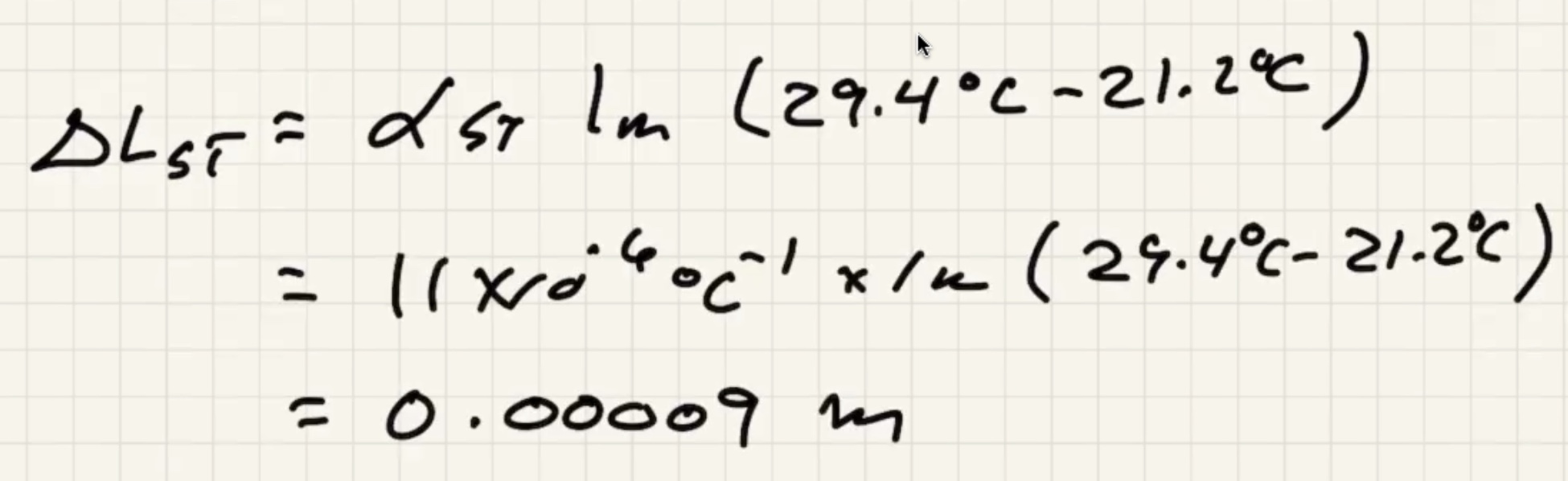
This means that the distance betweek meter marks on the tape is actually (1.00009 m)
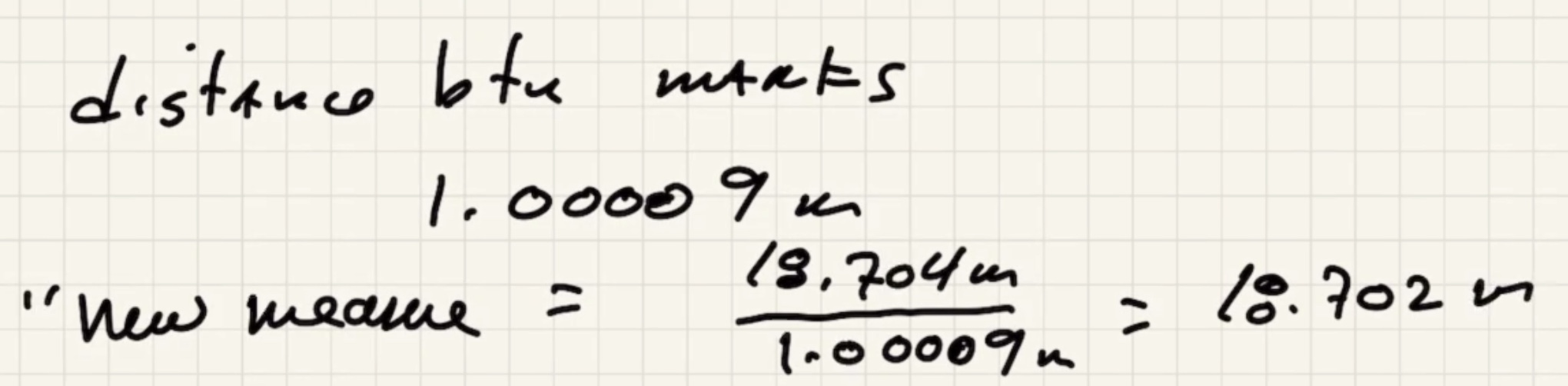
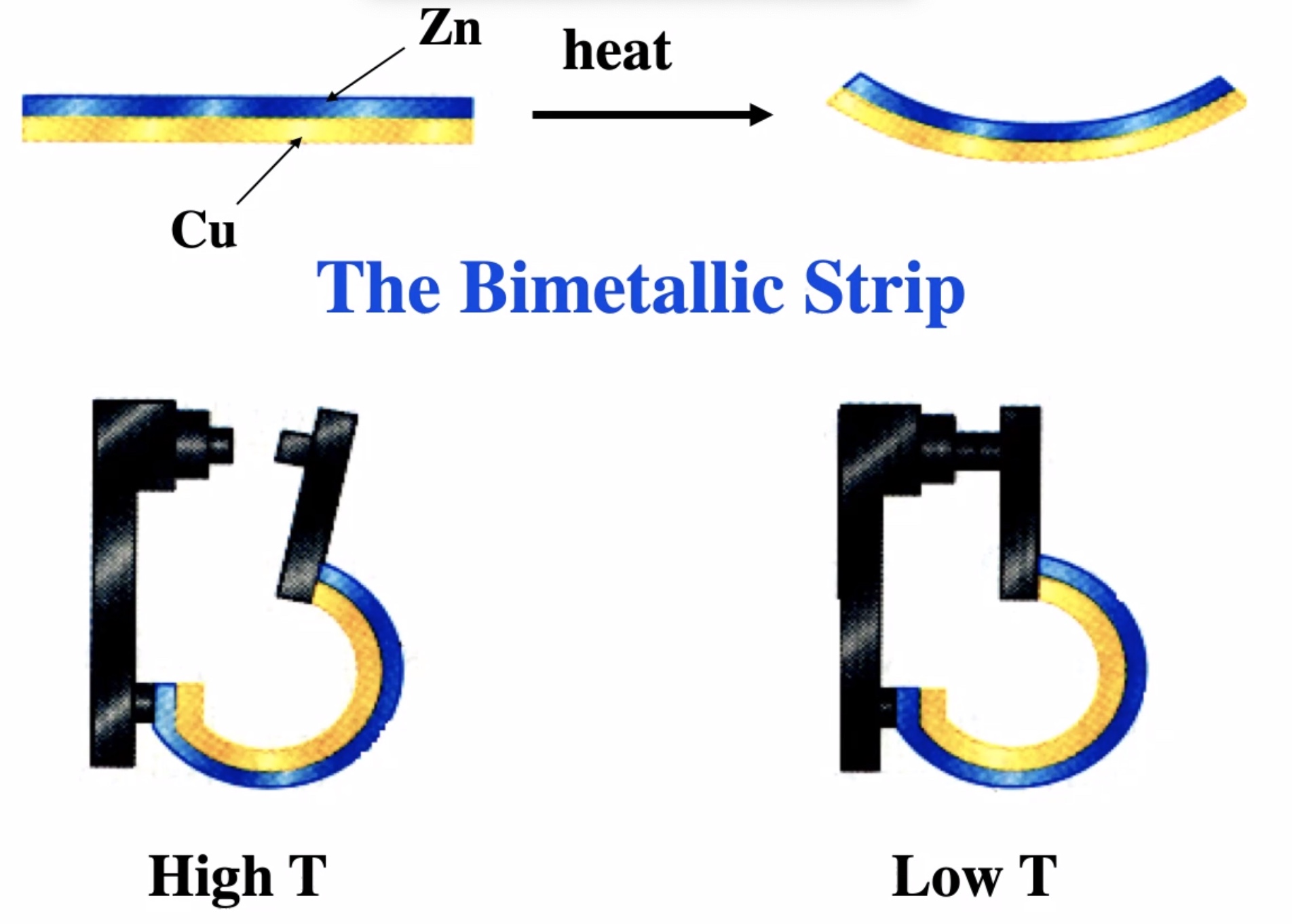
This is the way an old thermostat works:
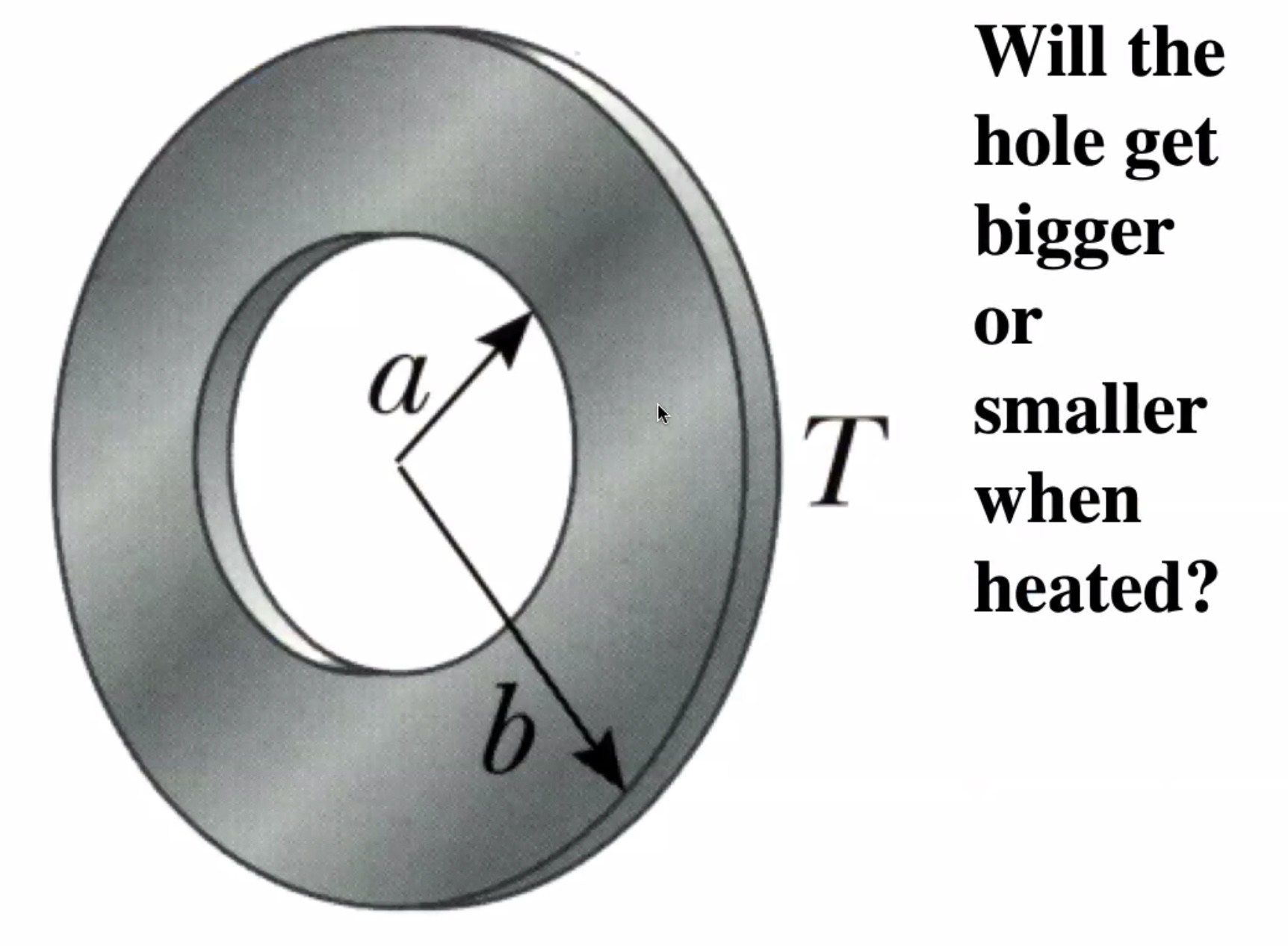
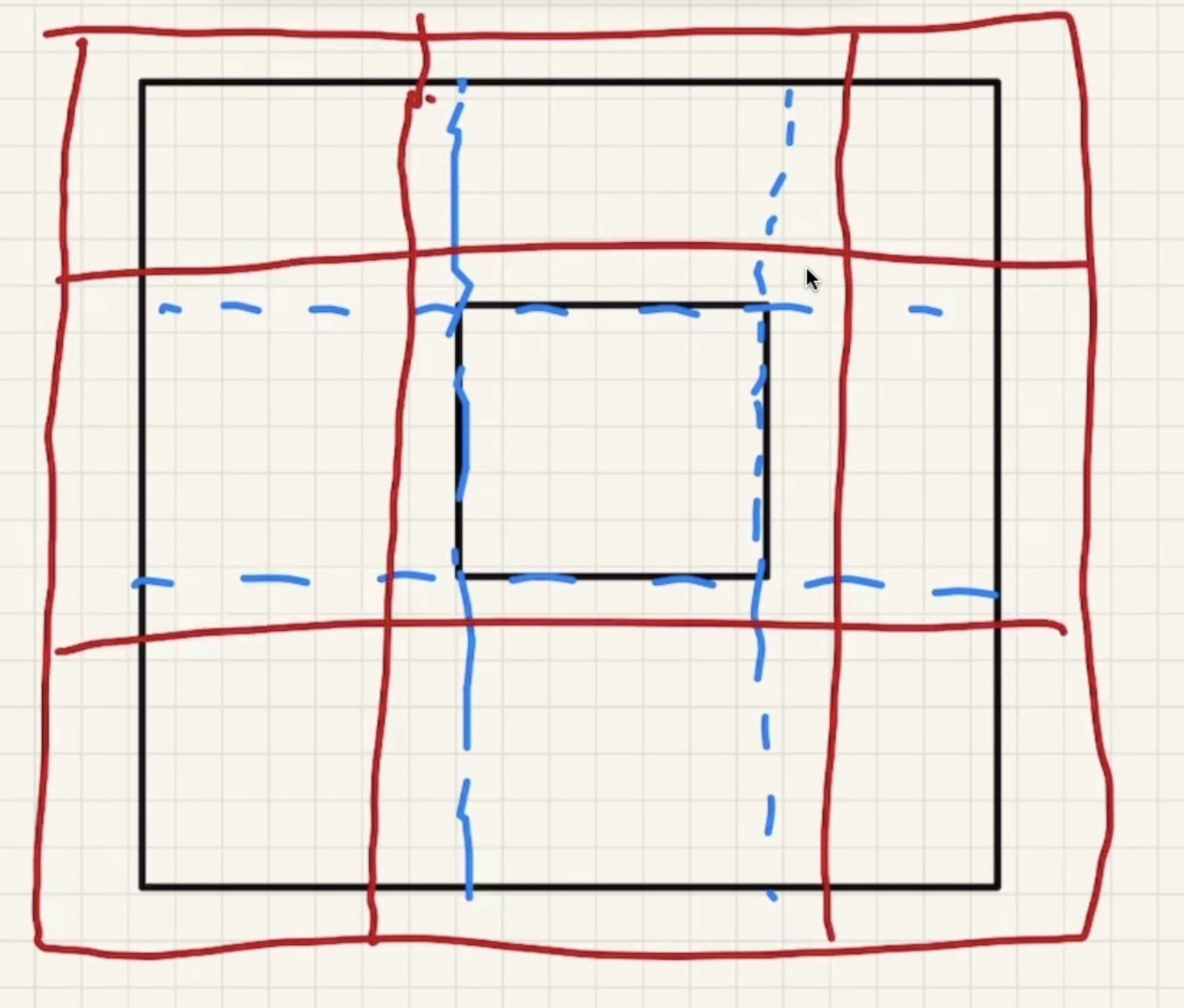
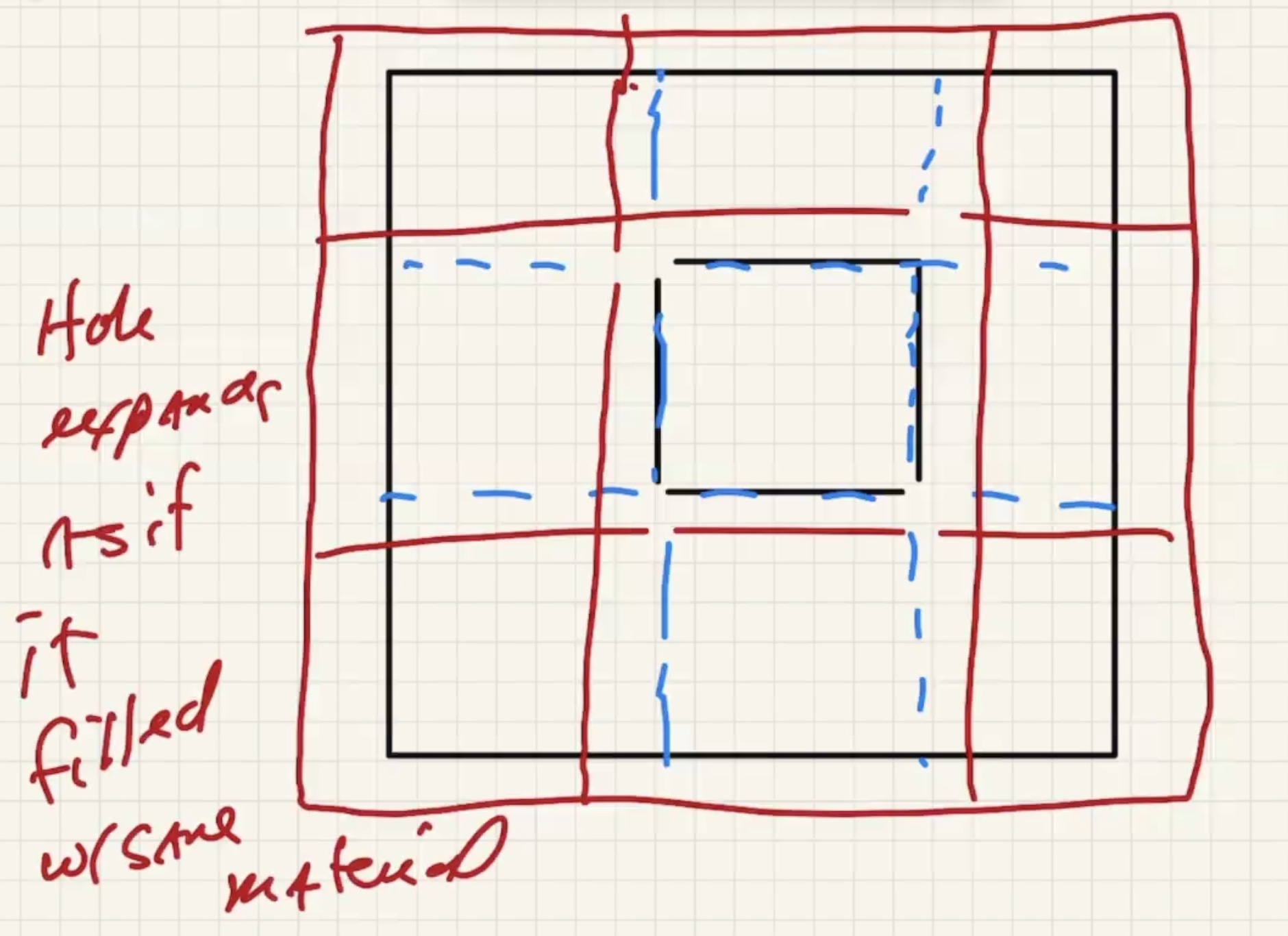
Lets consider a solid rectangular ring:
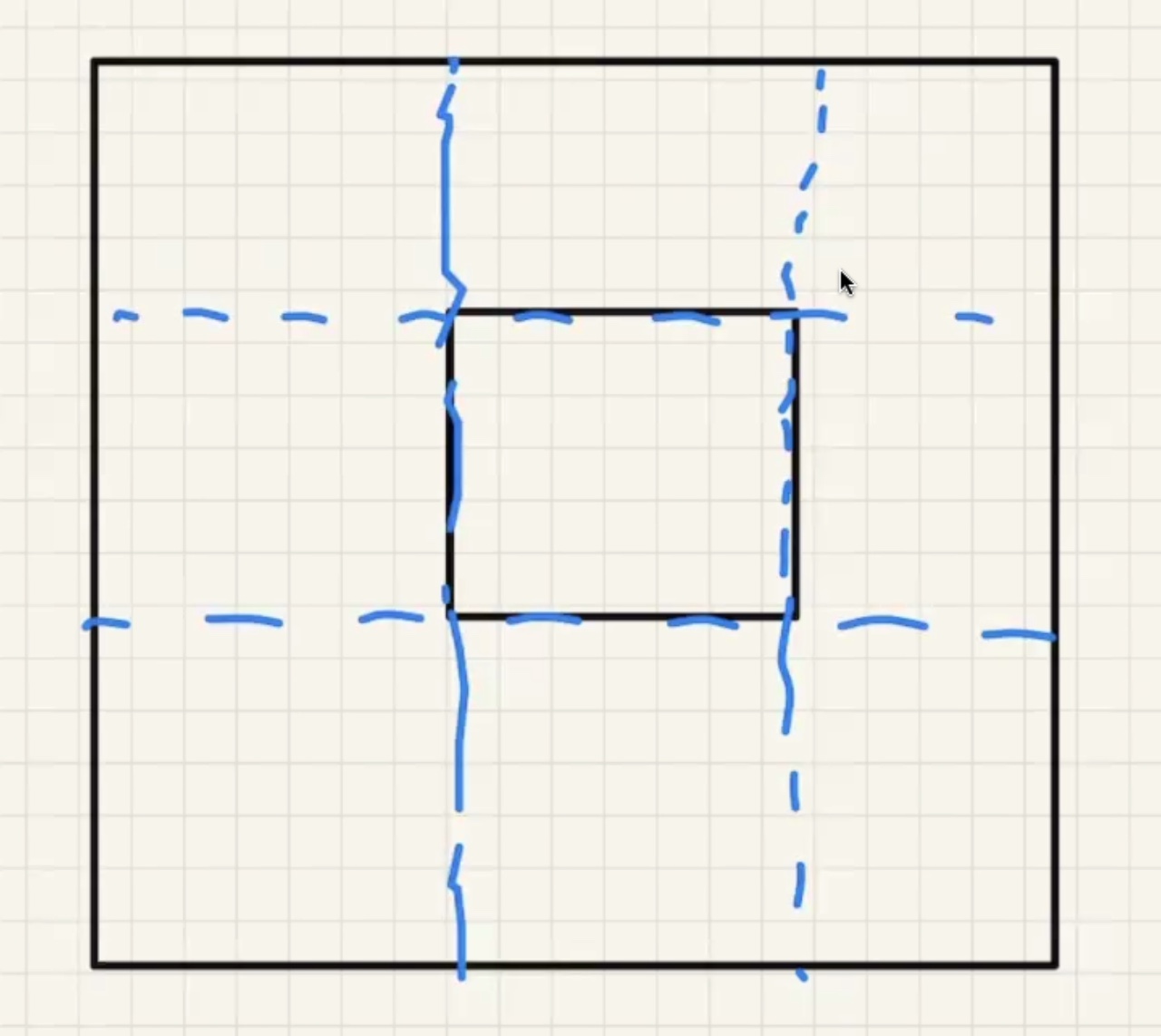
As the part heats up it expands: